Translate this page into:
Osteochondral lesions of the talus-current concepts

-
Received: ,
Accepted: ,
How to cite this article: Sundararajan SR, Dsouza TD, Rajagopalakrishnan R, Rajasekaran S. Osteochondral lesions of the talus-current concepts. J Arthrosc Surg Sports Med 2020;1(2):218-25.
Abstract
Osteochondral lesions of the talus encompass important clinical conditions encountered in day-to-day practice. Varied etiology and non-specific clinical signs make the diagnosis of these lesions challenging. Surgical treatment is indicated after a failed conservative trial, larger lesion and can be broadly split into cartilage repair, replacement, and regenerative strategies. Outcomes following surgery are variable and thus treatment strategy has to be tailored to every patient based on specific factors.
Keywords
Osteochondral lesions of the talus (OLT)
OCD talus
Osteochondritis dissecans
Microfracture
Cartilage repair techniques
INTRODUCTION
Osteochondral lesions of the talus (OLT) are those that affect the chondral and subchondral areas of the talus.[1] This is a broad terminology that encompasses a variety of disorders including osteochondritis dissecans, osteochondral fractures, and osteochondral defects.[2] Although majority may be associated with trauma, some may develop insidiously. These lesions pose a diagnostic challenge to the attending clinician due to lack of specific clinical signs and lack in consensus regarding treatment makes the management aspect controversial.[3] This review aims to elucidate the historical aspect of the disease, etiopathogenesis, classifications, diagnosis, and treatment to assist in day-to-day clinical practice.
HISTORY
The first description of osteocartilaginous loose bodies in the ankle, attributed to trauma, was given by Monro in 1738.[4] In 1922, Kappis extrapolated the concept of spontaneous necrosis at the hip to the etiopathogenesis of foreign bodies in the ankle joint and used the term osteochondritis dissecans.[5] In 1959, Berndt and Harty were the first to describe the pathogenesis of osteochondral lesions post-trauma. They also proposed the radiological classification that is widely employed even to the present day.[6] Kouvalchouk et al. in 1984 emphasized that these lesions should not be called as osteochondritis dissecans but be grouped under a broader term “osteochondral lesions of the talar dome.”[7] The arthroscopic treatment of these lesions was first described by Parisien and Pritsch et al. in 1986.[8,9] There have been numerous changes to the terminology of these lesions since the first description, however, the term “osteochondral lesions of the talus” (OLT) is generally preferred.[10]
ETIOPATHOGENESIS
Most OLT are secondary to trauma, with up to 50% of ankle sprains resulting in some grade of cartilage injury. Among the OLT, up to 94% of the lateral lesions are said to be secondary to trauma while only 62% of medial lesions are post-traumatic.[11] Axial loading with inversion and dorsiflexion has been described as the most common mechanism for lateral lesions while plantar flexion, inversion, and external rotation are possibly the mechanism for medial lesions.[10]
These repetitive injuries may result in microtrauma in an already vulnerable bone with sparse vascularity causing OLT. There are numerous reasons that make the talar cartilage and the subchondral bone prone to vascular insufficiency. First, the talar cartilage is relatively thinner with a thickness of 0.7–1.2 mm compared to that of other joints of the lower extremity.[12] Second, arterial supply to the talar dome and the overlying cartilage is by a retrograde vascular network that comes from the talar neck with additional watershed areas showing poor perfusion in the posteromedial, posterolateral, and mid-medial segments of the subchondral bone.[13] All these factors make the talus prone for developing osteochondral lesions.
CLINICAL FEATURES
Patients present with spectrum of non-specific complaints including of pain on weight-bearing, swelling, stiffness, and occasionally locking sensation at the ankle joint. A history of ankle trauma/recurrent instability is to be elicited as OLT are associated with ankle instability. Clinical examination may reveal effusion at the ankle, tenderness over the talus on palpation, decreased range of motion, and pain on ankle dorsiflexion and inversion.[14] Provocative tests such as anterior drawer test should be performed and compared to the unaffected side to evaluate the associated instability. This initial evaluation often leads to a broad differential diagnosis including ankle synovitis, impingement, occult fractures, and early ankle/subtalar arthritis.[2]
CLASSIFICATIONS
Plain radiography is the initial investigation of choice in a clinically suspected case of OLT. Berndt and Harty classification is the staging system that is widely employed for describing OLT on plain radiographs.[6] In case of clinically suspected lesion with negative radiographs, advanced imaging options such as CT and MRI are useful. MRI is the most sensitive imaging for OLT with a sensitivity of 96%.[15] Although it provides good visualization of the cartilage, it tends to overestimate the extent of the subchondral lesion due to the associated marrow edema. Hence, MRI is the investigation of choice in a clinically suspected lesion with negative radiographs while CT remains the preferred investigation for pre-operative planning with a positive plain radiograph as it better demonstrates the subchondral area of the lesion.[16] Ferkel et al. and Hepple et al. described the classifications of OLT based on CT and MRI, respectively.[17,18] Arthroscopy remains the gold standard as it allows direct visualization, probing of the lesion to assess the stability of the overlying cartilage and also accurately assess the extent of the lesion. Ferkel’s grading is the most commonly employed grading system on arthroscopy.[19] Commonly used classifications are summarized in [Table 1].
| Berndt and Harty classification[6] (based on radiographs) | Ferkel et al. classification[17](based on CT) | Hepple et al. classification[18] (based on MRI) |
Ferkel classification[19] (arthroscopic) |
|---|---|---|---|
| I: Subchondral impaction II: Partially detached osteochondral fracture III: Completely detached but undisplaced IV: Completely detached and displaced |
I: Cystic lesion at the dome with intact roof on all sides IIA: Cystic lesion with communication to talar dome surface IIB: Open articular surface lesion with overlying non-displaced fragment III: Undisplaced lesion with lucency IV: Displaced fragment |
IA: Articular cartilage damage only IIA: Cartilage injury with underlying fracture and surrounding bony edema IIB: Stage 2a without surrounding bony edema III: Detached but undisplaced fragment IV: Detached and displaced fragment V: Subchondral cyst formation |
A: Soft, smooth cartilage; B: Rough cartilage C: Fibrillation and fissures D: Flap present on exposed bone E: Loose fragment undisplaced F: Displaced fragment |
TREATMENT
Both non-operative and operative modalities have been described for the treatment of OLT.[5] The sole indication for operative treatment at presentation is an acute lesion with displacement.[20] Either fixation or excision is recommended for acutely displaced lesions while conservative treatment is the initial treatment indicated for all acute undisplaced lesions and other symptomatic OLT presenting late.[3] Conservative treatment mainly consists of rest, avoiding sporting activities, cast immobilization with or without NSAIDs, and intraarticular injection of platelet-rich plasma (PRP) and hyaluronic acid (HA). Rationale of conservative treatment is to offload the affected area for resolution of bone marrow edema and to facilitate healing of the detached cartilage. There are only a few recent studies that have reported the outcomes following non-operative management.[1] Klammer et al. described the natural history of OLT in 43 patients who opted for conservative treatment with a minimum follow-up of 2 years. They reported favorable outcomes with 86% of patients having no pain or only mild pain, no advancement of MRI staging in 84% of patients, and no significant ankle arthritis at final follow-up, though many patients reported minor discomfort on activities of daily living and sporting activities.[21] Weigelt et al. in their study of 22 patients with successful initial non-operative treatment of OLT reported only minimal symptoms, a low failure rate, and no significant progression of ankle arthritis at a minimum follow-up of 10 years, though a substantial number of patients (>1/3rd) reported a decrease in sporting activity.[22]
Injection therapy alone with PRP or HA has also been attempted in the treatment of OLT. HA is known to reduce inflammation in the joint while simultaneously substituting joint fluid. Growth factors contained in PRP can facilitate cartilage repair by stimulating matrix formation and increasing chondrocyte proliferation.[23] A level-II randomized study conducted by Mei-Dan et al. of 30 patients comparing intra-articular injection of PRP and HA concluded that both PRP and HA resulted in improved function and decreased pain that maintained for at least 6 months. Furthermore, PRP group had significantly better outcomes than the HA group.[24]
Operative treatment is indicated for OLT that have remained symptomatic even after a conservative trial for 3–6 months. Operative techniques can be broadly classified as cartilage repair, regeneration, and replacement techniques [Table 2].[2] Decision-making depends mainly on the stability of the overlying cartilage, size, and the containment of the lesion (shoulder and non-shoulder type lesion). A non-shoulder-type lesion is defined as a chondral defect that has surrounding articular cartilage (a contained cartilage defect), whereas a shoulder-type lesion does not have a peripheral cartilage border on one side with the loss of the medial or lateral articular buttress (uncontained defect).[25] Useful algorithm describing the indication for each procedure is outlined in [Figure 1].[2]
| Cartilage repair techniques | Cartilage replacement techniques | Cartilage regeneration techniques |
|---|---|---|
| Bone marrow stimulation/microfracture Retrograde drilling |
Osteochondral autograft transfer system Osteochondral allografts Particulated juvenile cartilage allograft transfer |
Autologous cartilage implantation Matrix-induced cartilage implantation Autologous matrix-induced chondrogenesis |
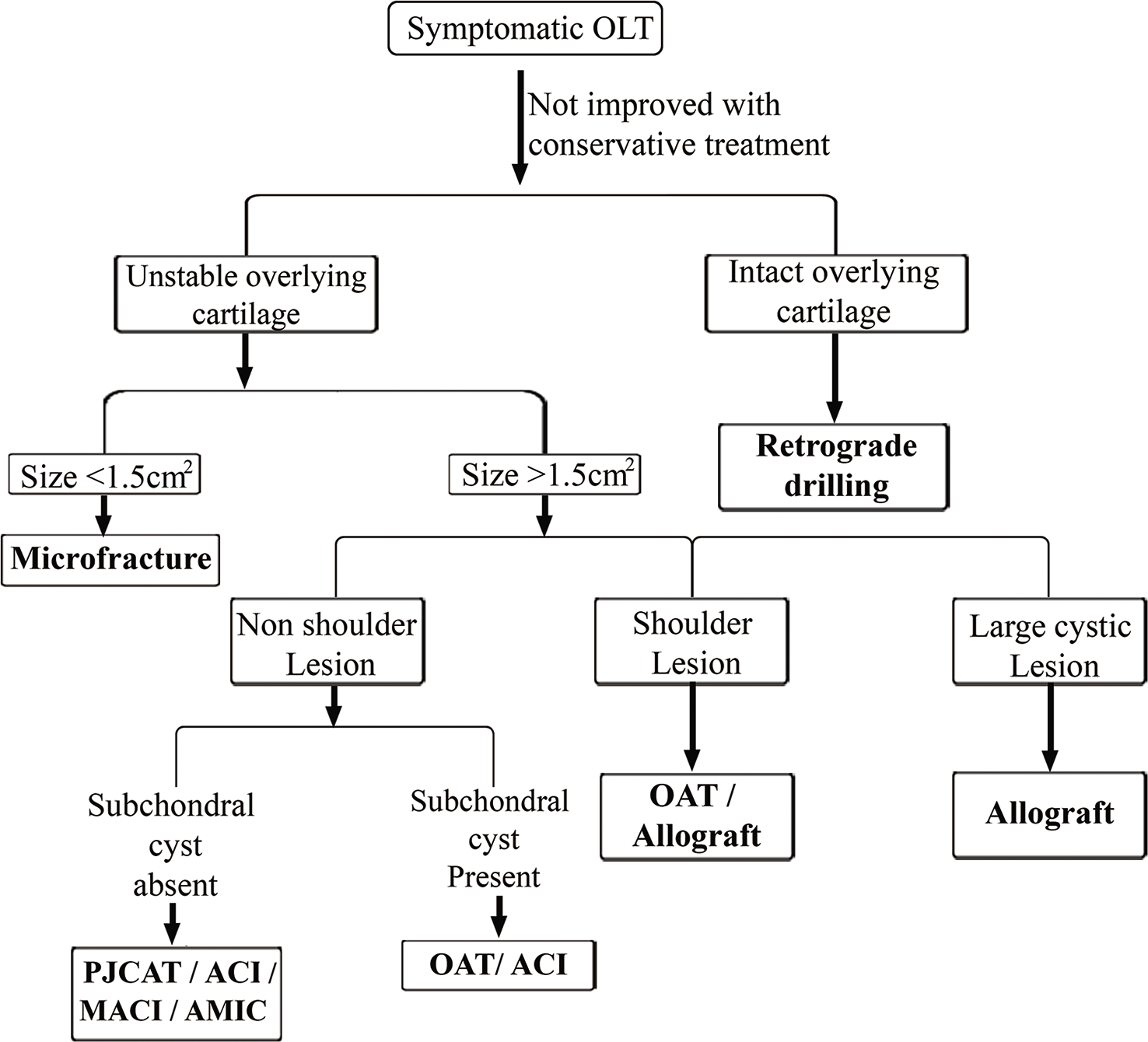
- Algorithm for the treatment of osteochondral lesions of the talus.
CARTILAGE REPAIR TECHNIQUES
Bone marrow stimulation/microfracture
Microfracture is a technique of perforating the subchondral bone to allow the progenitor cells from the bone marrow to infiltrate into the lesion [Figure 2]. These eventually would form fibrocartilage at the defect. Fibrocartilage is predominantly made of Type I collagen which is structurally and biomechanically inferior to hyaline cartilage.[26] Furthermore, the quantity and quality of fibrocartilage formed may vary. Yang and Lee revealed incomplete healing and inferior quality of cartilage in 36% (9/25) ankles in a second look arthroscopy analysis of arthroscopic microfracture at a mean follow-up of 3.6 years.[27] Toale et al. in their systematic review of 15 studies with a mean follow-up of 72 months also highlighted surface damage in 76% of patients on follow-up MRI that could be a harbinger for long-term problems.[28] Despite these findings, microfracture still seems to be resulting in good functional outcomes. Choi et al. in their study of 165 consecutive ankles with OLT demonstrated good functional outcomes and improved quality of life in patients at 6.7 years of follow-up.[29] Return to sports rate after microfracture is reported to be 76%, though most patients may not be able to achieve the pre-injury level.[30]
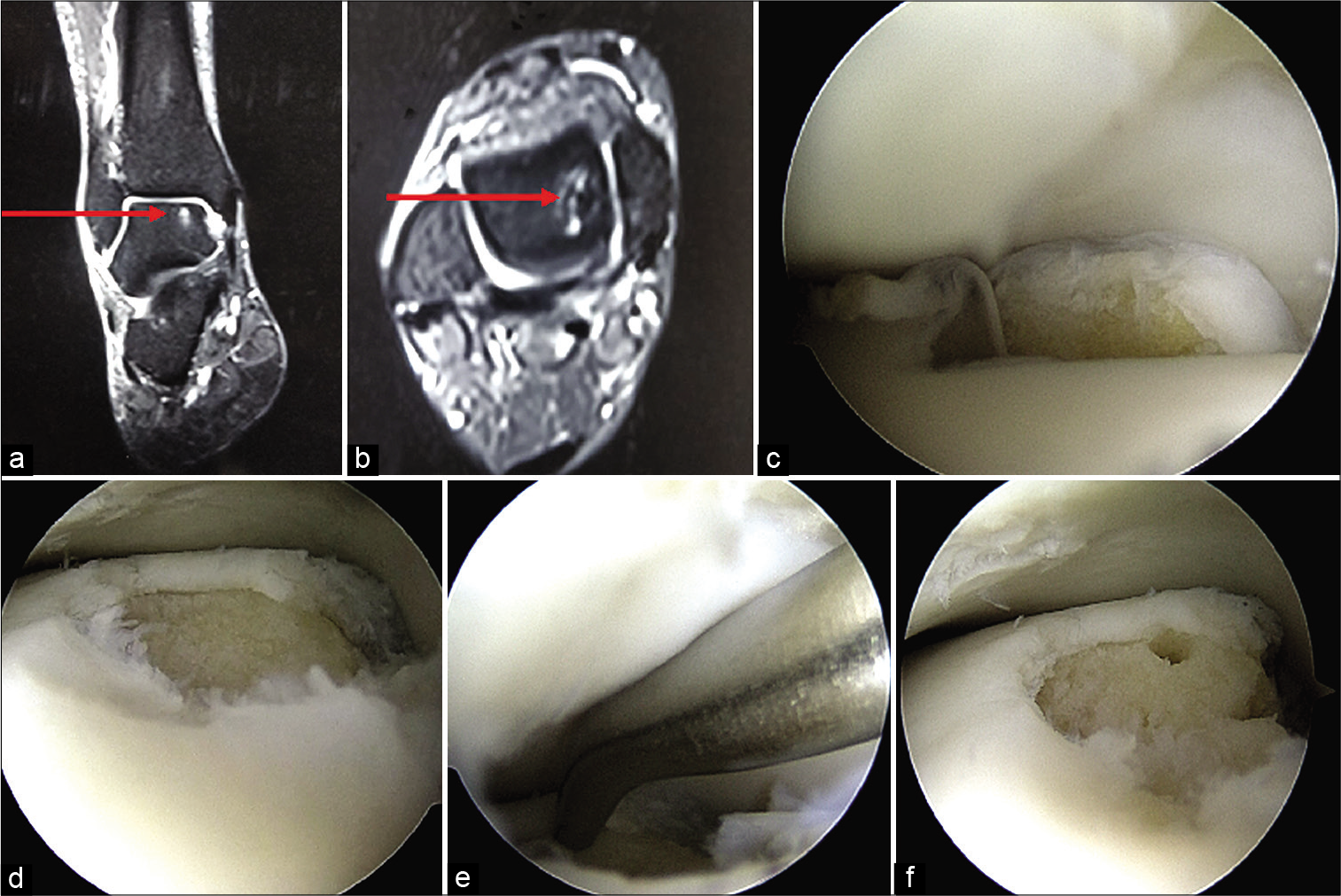
- Arthroscopic debridement and microfracture for osteochondral lesions of the talus (OLT). (a and b) MRI coronal and axial images (red arrow), (c) OLT with displaced cartilage, (d) post-debridement, (e) microfracture using microfracture pick, (f) completed procedure.
Polat et al. in their review of 82 patients reported increase in arthrosis by one grade radiologically though none of the patients had Grade IV arthritis at a minimum follow-up of 5 years.[31] The reported incidence of complication rate varies from 0 to 14% with superficial peroneal neuropathy and portal site pain cited as the most common complications.[28]
The prognostic factors that determine the success of microfracture are listed in [Table 3].[25,32-36]
| Good prognosis |
|---|
| Size of the lesion <1.5 cm2 |
| Acute lesions |
| Contained non-shoulder lesions |
| No prognostic significance |
| Age of the patient |
| Medial/lateral lesions, subchondral cysts |
Retrograde drilling
In OLT with subchondral cysts and intact overlying cartilage, retrograde drilling is a better modality that penetrates the necrosed subchondral bone without disturbing the overlying cartilage. Anders et al. reported retrograde drilling and autogenous bone grafting to be an excellent technique in their review of 41 patients of OLT with an intact overlying cartilage with good functional and radiological outcome.[37] Morphological evaluation post-retrograde drilling using second look arthroscopy showed no worsening of overlying cartilage at 1-year follow-up.[38]
CARTILAGE REPLACEMENT TECHNIQUE
Osteochondral autograft transfer system (OATS)
This technique involves harvesting osteochondral plugs from donor sites such as non-weight-bearing portions of the knee and implantation of these plugs to the areas of osteochondral defects. The main advantage of this procedure is that these harvested plugs are made up of hyaline cartilage (Type II collagen), restoring the articular cartilage to near normal. Donor site morbidity and the need for a medial malleolus osteotomy remain its major disadvantages.[2,39] Flynn et al. concluded OATS to be an effective treatment strategy even for large OLT with MOCART scoring showing good structural integrity of the graft at mean follow-up of 24.8 months and good functional outcomes irrespective of a prior microfracture or concomitant procedure.[40] A retrospective analysis of 131 patients suggested that though all patients returned to sporting activity, they engaged in fewer, less frequent sporting activities post-OATS treatment.[41]
Osteochondral allografting
This option is useful for large OLT with extensive subchondral cysts.[2] The allografts used are either fresh allografts which have to be used within 28 days or fresh frozen allografts with relatively less chondrocyte viability.[42,43] These grafts can then be employed for bulk transfer after size matching using CT scan.[2] This technique is effective in treating large cystic lesions even up to 6 cm2 with favorable outcomes reported.[44] A systematic review by Richard et al. reported a reoperation rate of 25% with development of moderate or severe ankle arthritic changes, pain due to hardware-related complications, graft collapse, and non-union/delayed union at osteotomy site as reasons for reoperation with a failure rate of 13.2%.[45] Due to high rates of reoperation and failure, it is necessary to opt for other less morbid techniques initially, keeping this technique of osteochondral allografting as a bail out procedure in failed cases. Availability of fresh donor allografts and the associated cost is also a concern in developing countries.
Particulated juvenile cartilage allograft transfer (PJCAT)
This is a relatively newer technique that employs transfer of particulated juvenile cartilage pieces with their native extracellular matrix harvested from deceased donors aged from newborns to 13 years. This is an US FDA approved allograft technique and was first made available in 2007 by DeNovo NT, Natural Tissue Graft (Zimmer, Inc., Warsaw. IN) to treat patellar lesion.[46] This harvested cartilage is then transferred to the area of defect and secured using fibrin glue. Particulated nature gives mobility to the chondrocytes to escape from the minced pieces and form a hyaline cartilage like matrix in the area of the defect. Furthermore, juvenile cartilage has been shown to possess copious cellular activity that results in formation of abundant extracellular matrix than its adult counterpart. Recommended indications include a symptomatic patient with size of the lesion at least 1.5 cm in one dimension or a patient who had a failed marrow stimulation technique. Lesions with large cystic areas, diffuse arthritic changes, ankle malalignment, and prior history of infections are contraindications to this procedure. This procedure is a single-stage procedure and as it does not require a press fit or graft contouring due to its particulate nature, it can be carried out arthroscopically.[46] As cartilage tissue is immune deprived, it is also not associated with immune reactions. Despite the advantages, the reported failure rate is 40% with lesions of area >125 mm2 and male sex associated with significantly higher risk of clinical failure. [47] A recent systematic review on the role of PJCAT in OLT involving 10 studies and 132 patients showed good postoperative functional outcomes, however, the regenerated cartilage was heterogeneous in nature with relatively unaltered subchondral area which is in contrast to the belief that PJCAT would restore the area of defect to near normal cartilage.[48]
CARTILAGE REGENERATION TECHNIQUE
Autologous chondrocyte implantation (ACI)
It is a 2-stage procedure where in hyaline cartilage is harvested either from the neck of the talus or non-weight portion of the knee joint, this cartilage is then cultured to grow chondrocytes which are implanted back to the area of defect and secured using a periosteal flap as a second-stage procedure.[2] Lee et al. studied the factors influencing the results of ACI in OLT and concluded that size >137 mm2 and age <26 years to be significantly associated with better MOCART (modified magnetic resonance observation of cartilage repair tissue) scores while patients sex, depth of the lesion, and presence or absence of accompanied procedure did not affect the results of ACI in OLT.[49]
Matrix-induced autologous chondrocyte implantation (MACI)
This is a second-generation technique that employs a collagen matrix instead of a periosteal sleeve to secure the chondrocytes. Although it still remains a 2-staged procedure, using a collagen matrix reduces the operative time and also helps in even distribution of chondrocytes.[50] Kreulen et al. reported good pain relief and function in a prospective study of 10 patients with a long follow-up of 13 years and concluded that MACI should be considered for osteochondral lesions that fail initial microfracture treatment.[51]
Autologous matrix-induced chondrogenesis
This technique combines microfracture with either autologous iliac crest bone marrow aspirate concentrate (BMAC) or PRP secured to the defect using a collagen scaffold/fibrin glue in a single stage [Figure 3].[2] The rationale is to form a hyaline cartilage at the defect from pluripotent cells instead of fibrocartilage that forms after microfracture alone. Guney et al. found PRP as an adjunct to arthroscopic microfracture for the treatment of OLT resulted in improved functional score status at an average follow-up of 16.2 months.[52] A systematic review of level 1 and 2 studies by Yausep et al. that included four studies concluded that PRP used in conjunction with microfracture results in better pain and functional improvement than microfracture alone. Furthermore, improvement was better when PRP was used as an adjunct to microfracture than a conservative intraarticular injection of PRP.[23] As far as BMAC is concerned, varying degrees of beneficial effects have been reported in different studies when used as an adjunct to surgical procedures. Chahla et al. in their review highlighted the paucity of long-term high-level studies regarding usage of BMAC in OLT with most evidence coming mainly from retrospective studies.[53]
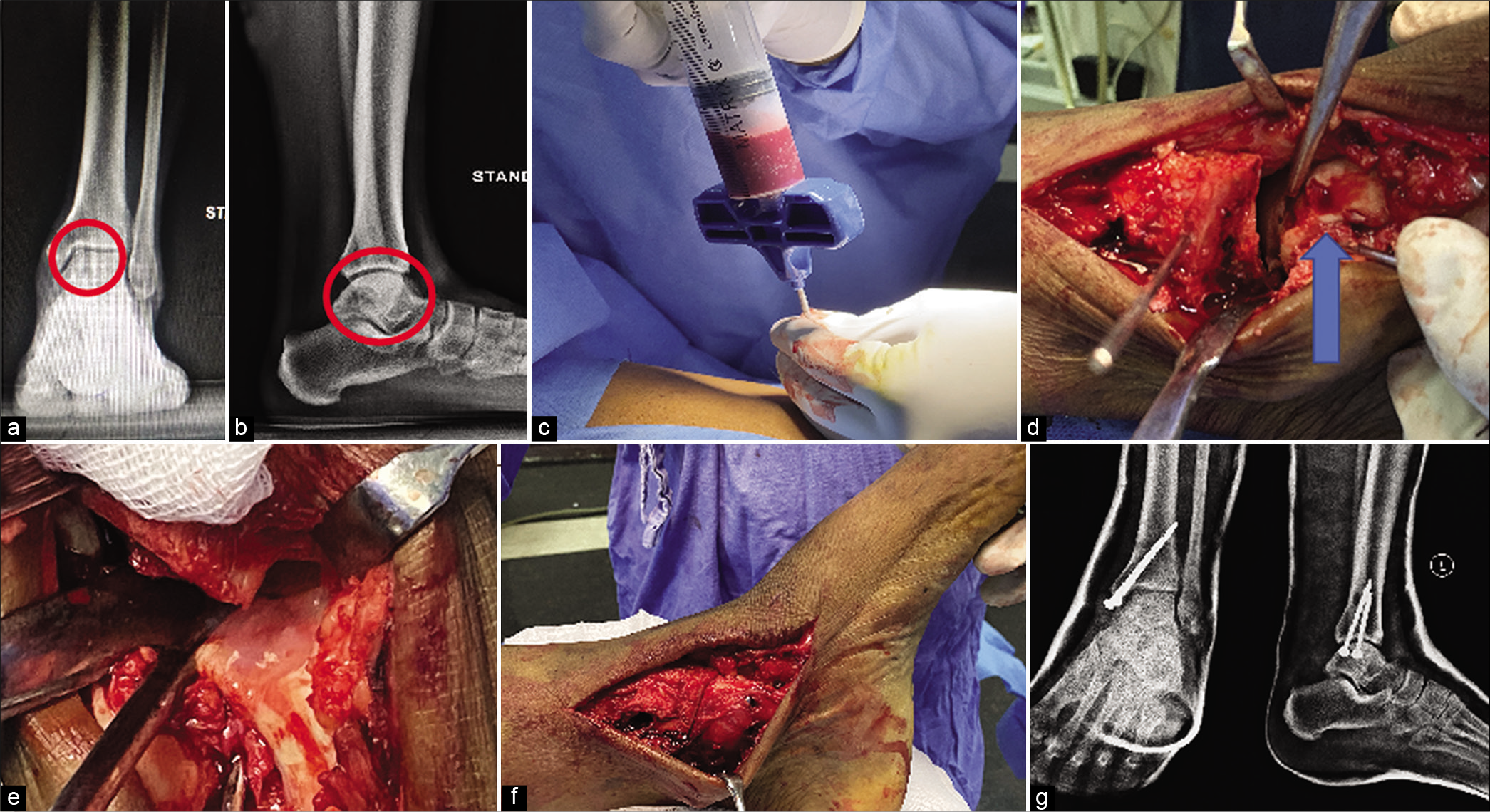
- Bone marrow aspirate concentrate (BMAC) for osteochondral lesions of the talus (OLT). (a and b) Plain radiographs showing OLT, where the red circled area depicts osteolytic lesion in talus located in postero-medial aspect of the talus. (c) aspiration of bone marrow from iliac crest, (d) medial malleolar osteotomy and demonstrating the lesion (blue arrow), (e) BMAC over the defect, (f) completed medial malleolar fixation using 4 mm cancellous screws, (g) post-operative radiograph.
In summary, outcomes following surgery are variable and thus treatment strategy has to be tailored to every patient depending on specific factors. A systematic review of 52 studies including 1236 primary OLT by Dahmen et al.[54] to detect the most effective treatment for OLT concluded that none of the interventions were clinically superior over another. They highlighted the heterogeneity of the data and suggested the need for high-quality prospective randomized studies using validated outcome measures for clarity regarding the effective modalities of treatment for OLT.[50]
CONCLUSION
OLT encompass a wide variety of disorders that are both difficult to diagnose and also to treat with varying functional outcomes. Clinicians should have a high index of suspicion as symptoms and clinical signs may be non-specific. Surgical technique should be mainly chosen depending on the status of the overlying cartilage, size, and containment of the lesion. Although outcomes of most of these techniques are promising, it is hard to recommend one procedure over another due to lack of comparative analyses. Thus, treatment should be individualized to every patient, with adequate counseling regarding the outcomes and associated complications of that technique.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Treatment of osteochondral lesions of the talus: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238-46.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral lesions of the talus: Current concepts in diagnosis and treatment. Foot Ankle Orthop. 2018;3:247301141877955.
- [CrossRef] [Google Scholar]
- Restorative tissue transplantation options for osteochondral lesions of the talus: A review. Orthop Clin North Am. 2017;48:371-83.
- [CrossRef] [PubMed] [Google Scholar]
- Part of the cartilage of the joint separated and ossified. Med Essays Obs. 1738;4:305.
- [Google Scholar]
- Weitere beitrage zur traumatisch-mechanischen entstehung der “spontanen” knorpelablösungen (sogen osteochrondritis dissecans) Dtsch Z Chir. 1922;171:13-29.
- [CrossRef] [Google Scholar]
- Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 2004;86:1336.
- [CrossRef] [PubMed] [Google Scholar]
- Lésions ostéochondrales du dôme astragalien avec nécrose partielle. Rev Chir Orthop. 1990;76:480-9.
- [Google Scholar]
- Arthroscopic treatment of osteochondral lesions of the talus. Am J Sports Med. 1986;14:211-7.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1986;68:862-5.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral lesions of the talus: Current concept. Orthop Traumatol Surg Res. 2010;96:554-66.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment strategies in osteochondral defects of the talar dome: A systematic review. Foot Ankle Int. 2000;21:119-26.
- [CrossRef] [PubMed] [Google Scholar]
- Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58:27-34.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative assessment of the subchondral vascularity of the talar dome: A cadaveric study. Foot Ankle Surg. 2014;20:57-60.
- [CrossRef] [PubMed] [Google Scholar]
- Current concept review: Osteochondral lesions of the talus. Foot Ankle Int. 2010;31:90-101.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondritis dissecans of the dome of the talus. Computed tomography scanning in diagnosis and follow-up. J Bone Joint Surg Am. 1988;70:1017-9.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic treatment of chronic osteochondral lesions of the talus: Long-term results. Am J Sports Med. 2008;36:1750-62.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral lesions of the talus: A revised classification. Foot Ankle Int. 1999;20:789-93.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral lesions of the talus. J Bone Joint Surg Am. 1980;62:97-102.
- [CrossRef] [Google Scholar]
- Natural history of nonoperatively treated osteochondral lesions of the talus. Foot Ankle Int. 2015;36:24-31.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus: An observational 14-year follow-up study. Orthop J Sports Med. 2020;8:2325967120924183.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet rich plasma for treatment of osteochondral lesions of the talus: A systematic review of clinical trials. J Orthop. 2020;18:218-25.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40:534-41.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of the containment and location of osteochondral lesions of the talus: Independent adverse outcomes associated with uncontained lesions of the talar shoulder. Am J Sports Med. 2013;41:126-33.
- [CrossRef] [PubMed] [Google Scholar]
- Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149-62.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic microfracture for osteochondral lesions of the talus: Second-look arthroscopic and magnetic resonance analysis of cartilage repair tissue outcomes. J Bone Joint Surg Am. 2020;102:10-20.
- [CrossRef] [PubMed] [Google Scholar]
- Midterm outcomes of bone marrow stimulation for primary osteochondral lesions of the talus: A systematic review. Orthop J Sports Med. 2019;7:2325967119879127.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic microfracture for osteochondral lesions of the talus: Functional outcomes at a mean of 6.7 years in 165 consecutive ankles. Am J Sports Med. 2020;48:153-8.
- [CrossRef] [PubMed] [Google Scholar]
- Return to sports after arthroscopic debridement and bone marrow stimulation of osteochondral talar defects: A 5-to 24-year follow-up study. Knee Surg Sport Traumatol Arthrosc. 2016;24:1311-5.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sport Traumatol Arthrosc. 2016;24:1299-303.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral lesion of the talus: Is there a critical defect size for poor outcome? Am J Sports Med. 2009;37:1974-80.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic treatment of osteochondral lesions of the talar dome: A retrospective study of 48 cases. Arthroscopy. 1999;15:77-84.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral lesion of the talus: Could age be an indication for arthroscopic treatment? Am J Sports Med. 2012;40:419-24.
- [CrossRef] [PubMed] [Google Scholar]
- Radiographic changes and clinical results of osteochondral defects of the talus with and without subchondral cysts. Foot Ankle Int. 2006;27:1109-14.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85:989-93.
- [CrossRef] [PubMed] [Google Scholar]
- Fluoroscopy-guided retrograde core drilling and cancellous bone grafting in osteochondral defects of the talus. Int Orthop. 2012;36:1635-40.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde drilling for osteochondral lesions of the talar dome. Am J Sports Med. 2006;34:1450-6.
- [CrossRef] [PubMed] [Google Scholar]
- Medial malleolar osteotomy for the correction of varus deformity during total ankle arthroplasty: Results in 15 ankles. Foot Ankle Int. 2008;29:171-7.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2016;37:363-72.
- [CrossRef] [PubMed] [Google Scholar]
- Return to sports after surgical treatment of osteochondral defects of the talus: A systematic review of 2347 cases. Orthop J Sports Med. 2019;7:2325967119876238.
- [CrossRef] [PubMed] [Google Scholar]
- Retrieved human allografts: A clinicopathological study. J Bone Joint Surg Am. 2001;83:971-86.
- [CrossRef] [Google Scholar]
- Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111-20.
- [CrossRef] [PubMed] [Google Scholar]
- Fresh osteochondral allograft for the treatment of cartilage defects of the talus: A retrospective review. J Bone Joint Surg Am. 2011;93:1634-40.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral allograft transfer for treatment of osteochondral lesions of the talus: A systematic review. Arthroscopy. 2017;33:217-22.
- [CrossRef] [PubMed] [Google Scholar]
- Particulated juvenile articular cartilage allograft transplantation for osteochondral lesions of the knee and ankle. Expert Rev Med Devices. 2020;17:235-44.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of particulated juvenile cartilage allograft transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2018;39:278-8.
- [CrossRef] [PubMed] [Google Scholar]
- Role of particulated juvenile cartilage allograft transplantation in osteochondral lesions of the talus: A systematic review. Foot Ankle Surg. ;2020
- [CrossRef] [PubMed] [Google Scholar]
- Factors influencing result of autologous chondrocyte implantation in osteochondral lesion of the talus using second look arthroscopy. Scand J Med Sci Sports. 2012;22:510-5.
- [CrossRef] [PubMed] [Google Scholar]
- Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85:109-15.
- [CrossRef] [PubMed] [Google Scholar]
- 13-year follow-up of treatment of osteochondral lesions with MACI. Foot Ankle Orthop. 2019;4:2473011419S0004.
- [CrossRef] [Google Scholar]
- Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23:2384-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow aspirate concentrate for the treatment of osteochondral lesions of the talus: A systematic review of outcomes. J Exp Orthop. 2016;3:33.
- [CrossRef] [PubMed] [Google Scholar]
- No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26:2142-57.
- [CrossRef] [PubMed] [Google Scholar]






