Translate this page into:
Evaluation of bone mineral density in cases of bone stress injury among sportspersons
*Corresponding author: Shubham Ahuja, Department of Sports Medicine, Sports Injury Centre, VMMC and Safdarjung Hospital, New Delhi, India. ahuja.aiims@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ahuja S, Jain V, Kataria H, Ghasi RG, Gupta H, Mittal M. Evaluation of bone mineral density in cases of bone stress injury among sportspersons. J Arthrosc Surg Sports Med 2022;3:72-7.
Abstract
Objectives:
A bone stress injury (BSI) represents the inability of bone to withstand repetitive mechanical loading, leading to structural fatigue, localized bone pain, and tenderness. Stress fractures account for up to 20% of athletic injuries and occur more commonly in women and track-and-field athletes. Low bone mineral density (BMD) has previously been considered a potential risk factor for developing BSI. This study aims to evaluate BMD, among other factors, in sportspersons diagnosed with BSI and look for association.
Materials and Methods:
Complete history and examination of a required sample size of 68 were done to reach a diagnosis of BSI, radiologically confirmed by magnetic resonance imaging (MRI). Diagnosed cases were tested for BMD by dual-energy X-ray absorptiometry (DEXA, whole body). Additional parameters studied included demographic profile, site of injury, MRI grade of injury, body mass index (BMI), and serum 25-hydroxy Vitamin D.
Results:
A total of 70 patients between the ages of 18 and 41 years with BSI were enrolled. Three subjects (4.29%) had low BMD. The mean BMD value was slightly lower in females (1.1 ± 0.07 vs. 1.19 ± 0.06 g/cm2 in males), but all three cases of low BMD were present among males. Additional findings included a high prevalence of low Vitamin D levels, male gender, low daily caloric intake, high-grade injuries on MRI, injuries at cortical-rich bone sites, and high BMI.
Conclusion:
Low BMD (Z-score<–1) prevalence of 4.29% suggests a lack of association with BSI. On the other hand, the relatively higher prevalence of Vitamin D insufficiency/deficiency, male gender, low daily caloric intake, high-grade injuries on MRI, and high BMI warrant attention.
Keywords
Bone stress injury
Bone mineral density
Stress fractures
Sportspersons
INTRODUCTION
A bone stress injury (BSI) represents the inability of bone to withstand repetitive mechanical loading, leading to structural fatigue, localized bone pain, and tenderness. [1] BSIs take place along a pathology continuum, starting with a stress reaction, progressing to a stress fracture, and finally a complete bone fracture.[1] First described in soldiers,[2] this type of fracture is commonly seen in runners, dancers, and other competitive athletes.[3] Stress fractures account for up to 20% of athletic injuries and occur more commonly in women and track-and-field athletes.[4,5] Anatomically, the tibia, fibula, and metatarsal bones are the most frequently affected sites.[6]
While an association of BSI with low bone mineral density (BMD) has not yet been established, some key intrinsic factors associated with increased risk of stress fractures, such as female sex,[7] hormonal irregularities, and low energy intake[8] are associated with low BMD as well. There is a conflicting evidence regarding the association between BSI and BMD. Some studies have found low BMD in athletes with stress fractures[9-11] while others have found no such correlation.[12,13]
We conducted this study to estimate BMD levels in diagnosed cases of BSI.
MATERIALS AND METHODS
This was an observational study conducted from October 2018 to April 2020 at a referral Sports Injury Centre in a medical college. Ethical clearance was taken from Institutional Ethics Committee. Patient selection was based on the following inclusion criteria: Age above 18 years, history of recent increase in physical activity leading to atraumatic bone pain, and evidence of BSI on magnetic resonance imaging (MRI). Exclusion criteria comprised cases with pathological fractures or a history of long-standing medical conditions/medications that influence bone health. Informed consent was obtained and the rights of subjects were protected.
Procedure
Complete history and physical examination of the selected patients were done to reach a provisional diagnosis of BSI, which was confirmed by MRI examination (Fredericson classification, graded 1–4).[14]
The patients thus diagnosed were tested for BMD by dual-energy X-ray absorptiometry (DEXA, whole body). The Resulting BMD values were standardized to T-scores and Z-scores with available age, weight, and ethnicity normative values of BMD. Low BMD was defined as a Z-score <–1.
Serum 25-hydroxy Vitamin D levels were determined. Vitamin D levels lower than 30 ng/ml were defined as low. Body mass index (BMI) was calculated using the formula: Weight (kg)/(height (m))2. The WHO chart and its revised criteria for the Asian population were employed to evaluate health status on the basis of calculated BMI. Daily caloric intake was estimated through the 24-h dietary recall method and energy availability (EA) was calculated. The association of BMD with the other variables collected was also assessed within the study group of BSI patients.
Sample size calculation
As observed in the study “Association of Hip BMD and Body Composition in a Rural Indian Population” by Mika Matsuzaki et al.,[15] the prevalence of low BMD in the Indian population from 20 to 29 years of age was approximately 20%. Taking these values as a reference, the minimum required sample size with 90% power of study, 5% level of significance, and 10% margin of error was 61.47. Accounting for the non-response rate, the total sample size came out to be 68.
Statistical analysis
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD and median. Normality of data was tested by Kolmogorov–Smirnov test. If the normality was rejected, then a non-parametric test was used. Quantitative variables were compared using the Independent t-test/Mann–Whitney test (when the data sets were not normally distributed) between the two groups and ANOVA/Kruskal–Wallis test was used for comparison between more than two groups. Qualitative variables were compared using Fisher’s exact test. P < 0.05 was considered statistically significant. The data were entered in the MS EXCEL spreadsheet and analysis was done using Statistical Package for the Social Sciences version 21.0.
RESULTS
A total of 70 patients, ranging from amateur to professional sportspersons, were enrolled in the study. The mean age at evaluation was 23.09 ± 5.3 years old (range 18–41 years old). Sixty-one out of 70 subjects were males.
Fifty-two of 70 (74.29%) study subjects had running as a primary sport, for instance, those in track-and-field events or marathons. These were differentiated from sportspersons whose sport involves running in addition to a primary skill (e.g., Football, basketball, and cricket, n = 18).
Seven cases (10%) had BSI at trabecular-rich sites, such as neck of femur (n = 4), sacrum (n = 1), and calcaneus (n = 1). Grade 3 MRI injuries (Fredericson classification) were most prevalent (32.86%, n = 23), closely followed by Grade 2 injuries (28.57%, n = 20). One out of every five patients (20%, n = 14) was Grade 4B (fracture line present) at the time of diagnosis.
The mean BMD was found to be 1.18 ± 0.06 g/cm2 while the mean Z-score was 0.21 ± 0.84 [Table 1, Figure 1]. Only three out of 70 subjects (4.29%) had low BMD.
| Bone mineral density | |
|---|---|
| Z-score | |
| Mean±SD | 0.21±0.84 |
| Median | 0.1 (–0.3–0.775) |
| Range | –1.6–2.7 |
| T-score | |
| Mean±SD | 0.01±0.73 |
| Median | –0.15 (–0.4–0.575) |
| Range | –1.5–2.4 |
| Total body bone mineral density (g/cm2) | |
| Mean±SD | 1.18±0.06 |
| Median | 1.18 (1.14–1.213) |
| Range | 1.01–1.36 |
SD: Standard deviation
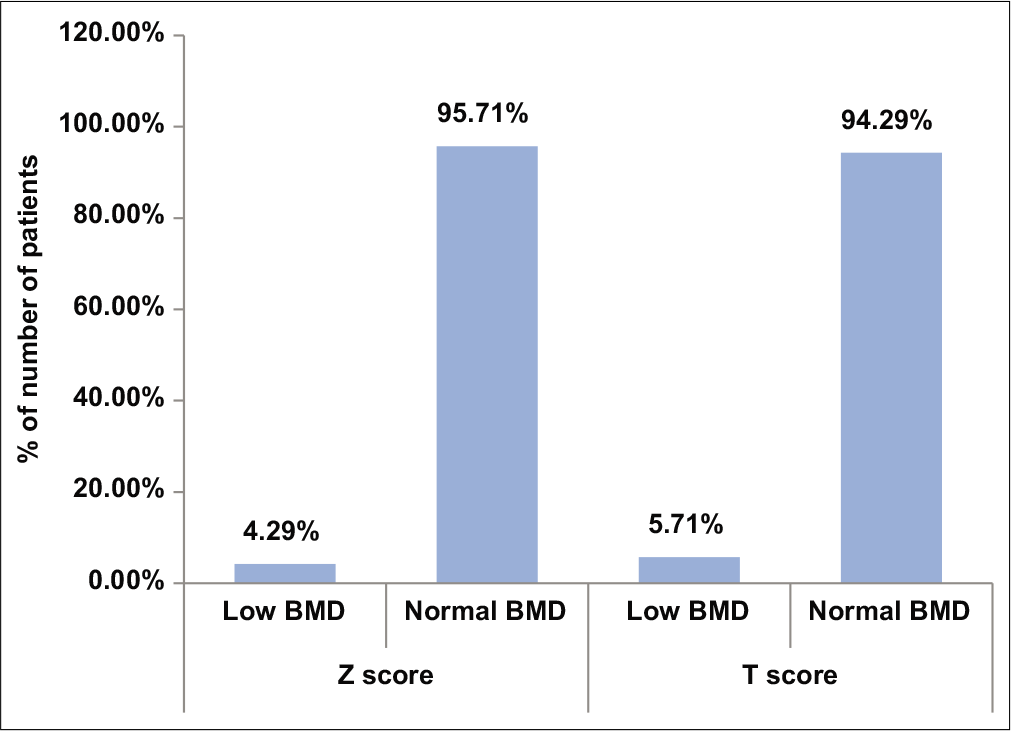
- Distribution of bone mineral density in the study subjects.
The mean BMD value was slightly lower in females (1.1 ± 0.07 vs 1.19 ± 0.06 g/cm2 in males, P < 0.0001), but all three cases of low BMD were present among males [Table 2, Figure 2]. Fifty-two (74.29%) subjects were found to have BMI in the normal range, 16 (22.86%) were either overweight or obese, and 2 (2.86%) were underweight.
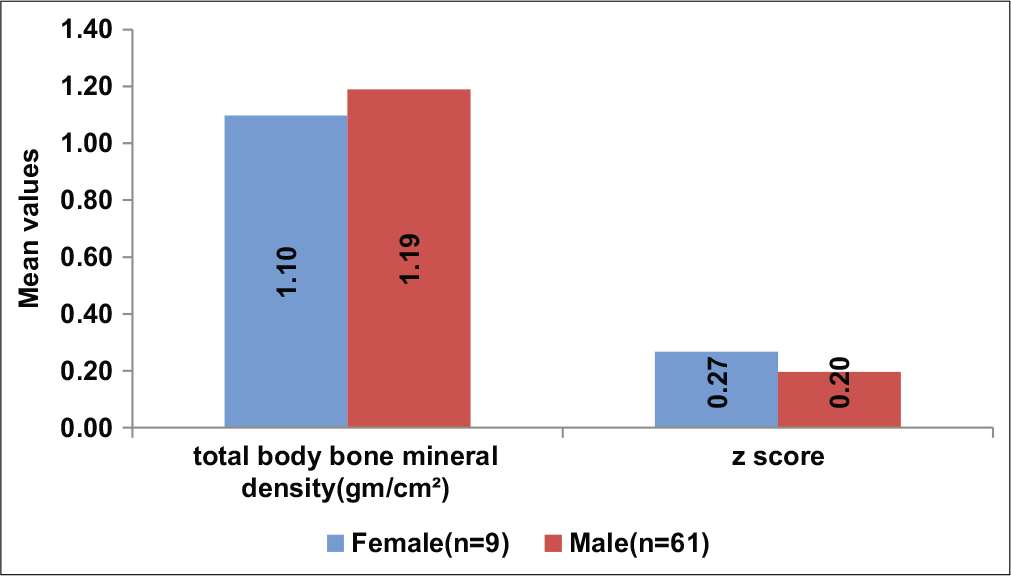
- Comparison of bone mineral density between genders.
| BMD | Female (n=9) | Male (n=61) | Total | P-value | Test performed | |
|---|---|---|---|---|---|---|
| Z score | ||||||
| Low BMD | 0 (0%) | 3 (4.92%) | 3 (4.29%) | 1 | Fisher Exact test | |
| Normal BMD | 9 (100%) | 58 (95.08%) | 67 (95.71%) | |||
| Mean±SD | 0.27±0.71 | 0.2±0.86 | 0.21±0.84 | 0.816 | t-test; 0.233 | |
| Median | 0.1 (–0.1–0.5) | 0.1 (–0.4–0.8) | 0.1 (–0.3–0.775) | |||
| Range | –0.6–1.8 | –1.6–2.7 | –1.6–2.7 | |||
| T score | ||||||
| Low BMD | 0 (0%) | 4 (6.56%) | 4 (5.71%) | 1 | Fisher Exact test | |
| Normal BMD | 9 (100%) | 57 (93.44%) | 66 (94.29%) | |||
| Mean±SD | 0.04±0.61 | 0±0.75 | 0.01±0.73 | 0.943 | Mann Whitney test; | |
| Median | –0.2 (–0.3–0.2) | –0.1 (–0.5–0.6) | –0.15 (–0.4–0.575) | 270.5 | ||
| Range | –0.5–1.5 | –1.5–2.4 | –1.5–2.4 | |||
| Total body bone mineral density (gm/cm2) | ||||||
| Mean±SD | 1.1±0.07 | 1.19±0.06 | 1.18±0.06 | <.0001 | t–test; 4.527 | |
| Median | 1.08 (1.064–1.11) | 1.19 (1.156–1.214) | 1.18 (1.14–1.213) | |||
| Range | 1.01–1.25 | 1.06–1.36 | 1.01–1.36 | |||
BMD: Bone mineral density, SD: Standard deviation
50% of cases were found to have low Vitamin D (n = 35). The mean Vitamin D level was 40.8 ng/ml (range 4.5–300.8 ng/ml) and the mean daily caloric intake was 2339 ± 233 kcal/day (range 1950–2800 kcal/day). None of our study subjects fulfilled the suggested energy allowance for Indian athletes.[16] No significant difference was found between runners and non-runners in the BMD values (1.17 ± 0.07 in runners vs. 1.19 ± 0.05 in non-runners; P = 0.3) and mean Z-scores (0.16 ± 0.89 vs. 0.34 ± 0.66; P = 0.4). The prevalence of low BMD was 5.77% in runners versus 0% in non-runners. However, this difference was not statistically significant (P = 0.6).
Subjects with injury in cortical-rich locations showed trends toward a higher prevalence of low BMD (4.76% vs. 0%) and lower mean Z-scores (0.16 vs. 0.6) than those in trabecular-rich locations, but the difference was not statistically significant (P = 1).
All subjects with low BMD had low Vitamin D levels and subjects with low vitamin D had lower mean Z-scores [Table 3, Figure 3].
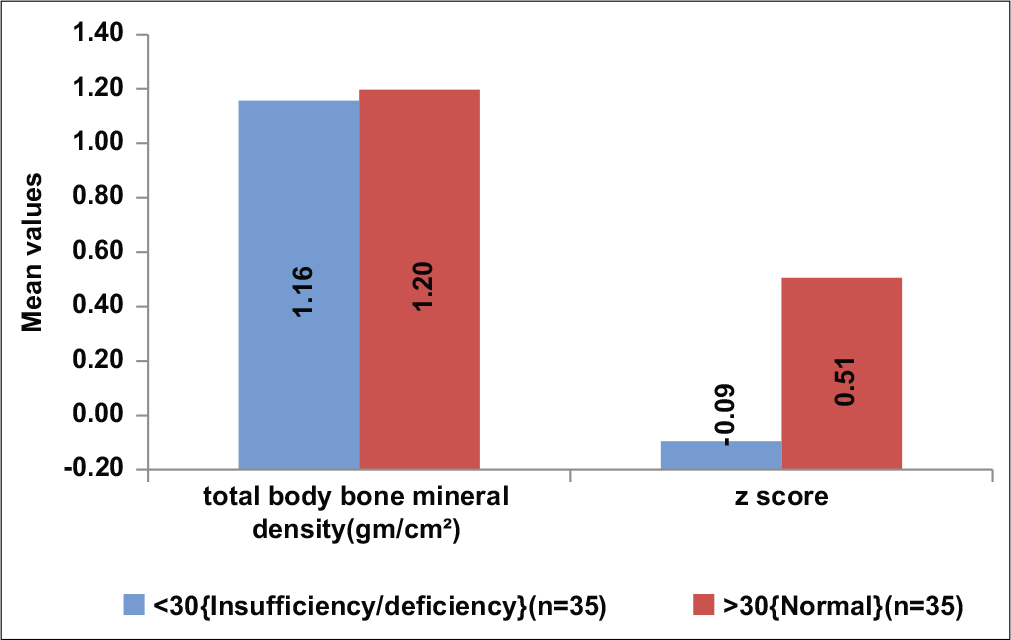
- Comparison of bone mineral density with Vitamin D level.
| Bone mineral density | <30 (Low) (n=35) | >30 (Normal) (n=35) | Total | P-value | Test performed |
|---|---|---|---|---|---|
| Z score | |||||
| Low BMD | 3 (8.57%) | 0 (0%) | 3 (4.29%) | 0.239 | Fisher Exact test |
| Normal BMD | 32 (91.43%) | 35 (100%) | 67 (95.71%) | ||
| Mean±SD | –0.09±0.72 | 0.51±0.84 | 0.21±0.84 | 0.002 | t-test; 3.197 |
| Median | –0.2 (–0.5–0.4) | 0.4 (–0.15–0.9) | 0.1 (–0.3–0.775) | ||
| Range | –1.6–1.7 | –0.7–2.7 | –1.6–2.7 | ||
| T score | |||||
| Low BMD | 4 (11.43%) | 0 (0%) | 4 (5.71%) | 0.114 | Fisher Exact test |
| Normal BMD | 31 (88.57%) | 35 (100%) | 66 (94.29%) | ||
| Mean±SD | –0.25±0.62 | 0.26±0.75 | 0.01±0.73 | 0.004 | MannT Whitney test; |
| Median | –0.3 (–0.65–0.15) | 0.1 (–0.2–0.65) | –0.15 (–0.4–0.575) | 371 | |
| Range | –1.5–1 | –0.8–2.4 | –1.5–2.4 | ||
| Total body bone mineral density (gm/cm2) | |||||
| Mean±SD | 1.16±0.06 | 1.2±0.06 | 1.18±0.06 | 0.007 | t-test; 2.743 |
| Median | 1.17 (1.128–1.2) | 1.2 (1.162–1.223) | 1.18 (1.14–1.213) | ||
| Range | 1.01–1.29 | 1.06–1.36 | 1.01–1.36 |
BMD: Bone mineral density, SD: Standard deviation
Subjects with high BMI (≥25 kg/m2, n = 6) had 33% prevalence of low BMD (P = 0.042) and had significantly lower mean Z-scores (–0.68 ± 0.69) than those with normal BMI (0.32 ± 0.85, P = 0.044, F value=2.84) [Table 4, Figure 4]. There were no significant differences on comparing the mean daily caloric intake of subjects with low BMD (2356.67 ± 273.01 kcal/day) and those with normal BMD (2338.6 ± 234.13 kcal/day, P = 0.896).
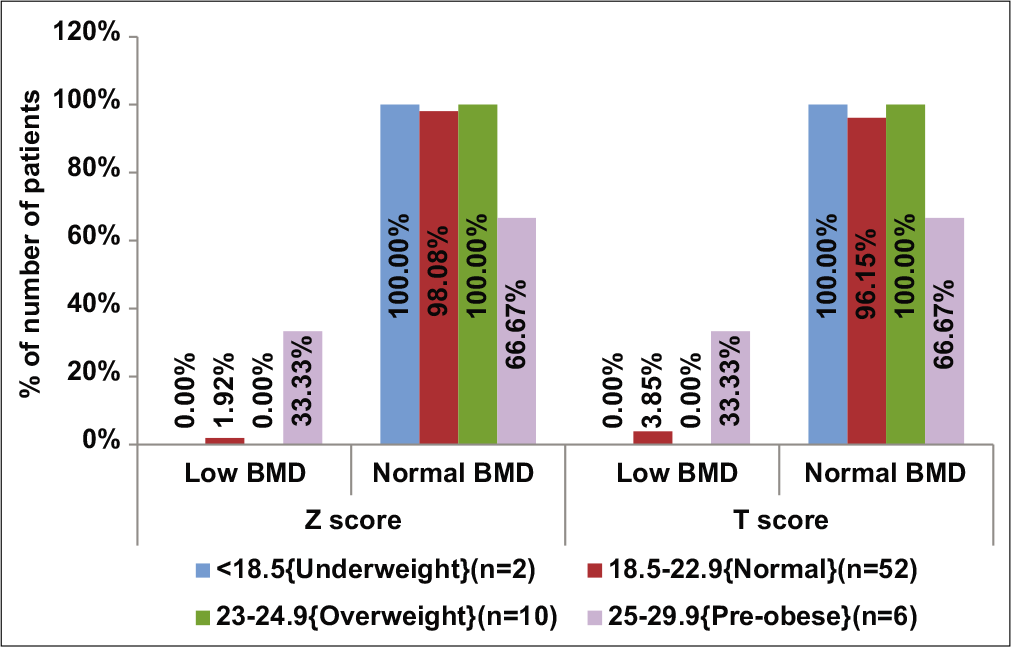
- Distribution of bone mineral density between body mass index groups (kg/m2).
| Body mass index (kg/m2) | Frequency | Percentage |
|---|---|---|
| <18.5 (Underweight) | 2 | 2.86% |
| 18.5–22.9 (Normal) | 52 | 74.29% |
| 23–24.9 (Overweight) | 10 | 14.29% |
| 25–29.9 (Pre-obese) | 6 | 8.57% |
| Mean±SD | 21.65±2.29 | |
| Median | 21.5 (19.925–22.8) | |
| Range | 17.3–28.1 | |
DISCUSSION
BSIs frequently occurs in athletes and cause a considerable loss of participation time. Around one-third to two-thirds of runners have a history of BSI, with at least half of them having a recurrence on more than one occasion.[9,17,18]
BMD, the inorganic content of bone, is one of the potential risk factors of BSI. Low BMD is commonly associated with osteoporosis and increased fracture risk, due to decreased bone strength. In our observational study, low BMD (defined as Z-score <–1) was found in three out of 70 subjects diagnosed with BSI. Considering the fact that the prevalence of low BMD has been reported to be 20% in the Indian population,[15] these data suggest a lack of association between BSI and low BMD.
The BMD findings of our study are in contrast to a study by Tenforde et al., in which a positive correlation was found between BSI at trabecular-rich locations and low BMD.[19] The contrasting findings might be explained by the lower sample size in that study and by the fact that those authors had selected their sample from athletes referred for a bone health evaluation, thus possibly increasing the overall prevalence of low BMD.
Myburgh et al.[10] in a case-control study also found the BMD of athletes with stress fractures to be lower than that of well-matched and uninjured athletes.
Our BMD results support the claim of Giladi et al.[13] regarding the lack of a relationship between osteoporosis and stress fractures. The finding was considered “not surprising from a biomechanical perspective,” since neither bone mineral content nor BMD influences bending forces on a bone. This study instead found narrow tibial width and increased degree of hip external rotation to be risk factors for stress fractures.
Frusztajer et al.[12] found no difference in BMD of ballet dancers and male Israeli military recruits with stress fractures and those of matched controls. Another case-control study, performed by Lauder et al. in 2000 to examine the role of BMD among 185 active-duty female soldiers, showed a strong negative relationship between the probability of stress fractures and BMD of the femoral neck.[20]
The results provide new insight into the concept of required bone density for physically active people and sportspersons, especially runners. Bennell et al.[18] had reported that even though the BMD of athletes with stress fractures was lower than that of matched athletes without stress fractures, it was still higher than that of matched uninjured non-athletes. Hence, it was suggested that the level of bone density required by sportspersons for optimum bone health is greater than that required by the general population.
Looking at the demographic profile of our study, the majority of cases were sportspersons whose primary sport is running. This is an expected finding since most of the previous research on BSIs has shown runners to be the most affected group.[3,19]
Classifying on the basis of site of injury, cortical-rich locations represented 90% of study subjects. Tenforde et al. also showed a higher prevalence of BSI at cortical-rich locations.[19] However, the above-mentioned study showed a higher risk for low BMD in trabecular-rich BSIs, while our study shows a higher prevalence of low BMD in cortical-rich BSIs.
The gender ratio of BSI in our study was heavily in favor of males (87.14%) and although females showed lower mean BMD scores, all the cases of low BMD (osteopenia) were male. This goes against the historical prevalence of BSIs, dominated by females, but reinforces the current belief that the pathophysiology of this condition, possibly starting from low EA, affects males equally.[3,21,22]
Our study also showed half of the study subjects to be having low Vitamin D levels. This was not surprising since the prevalence of Vitamin D deficiency in India has been reported to be ranging from 50% to 90% in hospital-based and community-based studies.[23] The mean BMD values were significantly lower in subjects with low Vitamin D levels.
A retrospective cohort study on confirmed stress fracture cases by Miller et al. showed Vitamin D levels to be below 40 ng/ml in 44 out of 53 subjects.[24] While our study did show a prevalence of 50% for low Vitamin D levels among BSI cases, the relevance of the same is equivocal, given the high prevalence of Vitamin D deficiency in the general population.
The other noteworthy finding of the study was the daily caloric intake of our subjects. The energy allowance suggested for Indian endurance athletes is 5200 kcal/day for an athlete weighing 65 kg (80 kcal/kg/day).[16] None of our study subjects fulfilled this value, pointing toward a highly prevalent state of low energy availability (EA).
Low EA is an established concept in sports medicine, defined as the failure to achieve sufficient energy intake to cover the energy spent on physical activity in addition to basic metabolic function. Low EA has been reported in endurance athletes and is now being considered an important cause of athlete illness and injury.
Earlier, low EA was studied primarily in female athletes and was considered a component of the female athlete triad.[25,26] Studies in recent years, however, have shown the negative effects on male athletes as well. The concept of Relative Energy Deficiency in Sports (RED-S) was thus introduced, to include both sexes and a broader spectrum of health and performance-related concerns as a consequence of low EA.[21] These athletes suffer from health issues that parallel the triad of low EA, hypogonadotropic hypogonadism, and low BMD. Low EA has been shown to have a negative effect on bone turnover and is associated with up to 4.5 times the increased rate of bone injury.[27,28] It had also been shown to be the main cause of suppression of metabolic and reproductive hormones in female athletes.[29] As found in our study, Melin et al.[30] also found a higher incidence of career BSI in males with low EA, despite having no difference in BMD.
The prevalence of high BMI in the present BSI cohort was another noteworthy finding. About 23% of our subjects had BMI in the overweight category or above (>23 kg/m2) while only two subjects (approximately 3%) were in the underweight category. Lauder et al. commented on the relationship of high BMI with BMD and stress fractures, stating that while high BMI had a beneficial effect on bone density, it also increased the probability of stress injury.[20]
Our study acknowledges certain limitations. Females constituted <13% of our study subjects. However, this was representative of the patients reporting to and diagnosed with BSI at our place of study and not a result of selection bias. The sample size, calculated to be adequate for the primary objective, was too small for studying the association with certain secondary factors such as BMI. The reliability of data on daily caloric intake might have been impacted by the recall bias of subjects. Finally, our study design is constrained by the lack of comparison with uninjured athletes or matched control groups.
The relatively higher prevalence of low Vitamin D levels and low EA found in our study require further investigation.
CONCLUSION
The prevalence of low BMD (Z-score <–1) in patients with BSI was 4.29%, suggesting a lack of association with BSI. On the other hand, the relatively higher prevalence of Vitamin D insufficiency/deficiency, male gender, low daily caloric intake, high-grade injuries on MRI, and high BMI warrant attention. Further research should be directed at studying these factors and their role in the pathology continuum of stress fractures and appropriate interventions to modify them for injury prevention as well as treatment.
Acknowledgments
All authors participated in the concept and design of the study. RGG and MM participated in data acquisition. VJ and HG contributed to data acquisition, data analysis, and statistical analysis. SA contributed to all steps of the study. All authors participated in manuscript editing and review.
We thank our institution for the smooth running of our study. None of the authors have a conflict of interest.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical Sports Medicine: Injuries. In: 5th ed. Vol 1. Chennai, Tamil Nadu: McGraw Hill; 2017.
- [Google Scholar]
- Epidemiology of stress fracture injuries among US high school athletes, 2005-2006 through 2012-2013. Am J Sports Med. 2015;43:26-33.
- [CrossRef] [PubMed] [Google Scholar]
- Females have a greater incidence of stress fractures than males in both military and athletic populations: A systemic review. Mil Med. 2011;176:420-30.
- [CrossRef] [PubMed] [Google Scholar]
- Update on stress fractures in female athletes: epidemiology, treatment, and prevention. Curr Rev Musculoskelet Med. 2013;6:173-81.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of stress fractures: The fundamentals. Clin Sports Med. 2006;25:29-36.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic factors for exercise-related injuries among male and female army trainees. Am J Sports Med. 1993;21:705-10.
- [CrossRef] [PubMed] [Google Scholar]
- Menstrual irregularity and stress fractures in collegiate female distance runners. Am J Sports Med. 1988;16:209-16.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007;39:1457-63.
- [CrossRef] [PubMed] [Google Scholar]
- Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113:754-9.
- [CrossRef] [PubMed] [Google Scholar]
- Stress fractures and bone health in track and field athletes. J Sci Med Sport. 2000;3:268-79.
- [CrossRef] [Google Scholar]
- Nutrition and the incidence of stress fractures in ballet dancers. Am J Clin Nutr. 1990;51:779-83.
- [CrossRef] [PubMed] [Google Scholar]
- Stress fractures. Identifiable risk factors. Am J Sports Med. 1991;19:647-52.
- [CrossRef] [PubMed] [Google Scholar]
- Tibial stress reaction in runners: Correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23:472-81.
- [CrossRef] [PubMed] [Google Scholar]
- Association of hip bone mineral density and body composition in a rural Indian population: The Andhra Pradesh Children and Parents study. PLoS One. 2017;12:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- International Life Sciences Institute-India. National Institute of Nutrition, Sports Authority of India. In: Nutrition and Hydration Guidelines for Excellence in Sports Performance. New Delhi, India: International Life Sciences Institute-India; 2007.
- [Google Scholar]
- Risk factors for stress fractures in female track-and-field athletes: A retrospective analysis. Clin J Sports Med. 1995;5:229-35.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and distribution of stress fractures in competitive track-and-field athletes: A twelve-month prospective study. Am J Sports Med. 1996;24:211-7.
- [CrossRef] [Google Scholar]
- Low bone mineral density is associated with bone stress injuries at anatomic sites with greater trabecular composition. Am J Sports Med. 2018;46:30-6.
- [CrossRef] [PubMed] [Google Scholar]
- The relation between stress fractures and bone mineral density: evidence from active-duty Army women. Arch Phys Med Rehabil. 2000;81:73-9.
- [CrossRef] [PubMed] [Google Scholar]
- The IOC consensus statement: Beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S) Br J Sports Med. 2014;48:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- Parallels with the female athlete triad in male athletes. Sports Med. 2016;46:171-82.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D deficiency in India. J Family Med Prim Care. 2018;7:324-30.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Vitamin D with stress fractures: A retrospective cohort study. J Foot Ankle Surg. 2016;55:117-20.
- [CrossRef] [PubMed] [Google Scholar]
- 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the Female Athlete Triad: 1st International Conference held in San Francisco. California May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48:289.
- [CrossRef] [PubMed] [Google Scholar]
- Female athlete triad and its components: Towards improved screening and management. Mayo Clin Proc. 2013;88:996-1009.
- [CrossRef] [PubMed] [Google Scholar]
- Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in long distance athletes. Int J Sport Nutr Exerc Metab. 2018;28:403-11.
- [CrossRef] [PubMed] [Google Scholar]
- Induction and prevention of low-T3 syndrome in exercising women. Am J Physiol. 1993;264:924-30.
- [CrossRef] [PubMed] [Google Scholar]
- Low energy availability in the marathon and other endurance sports. Sports Med. 2007;37:348-52.
- [CrossRef] [PubMed] [Google Scholar]
- Energy availability in athletics: Health, performance and physique. Int J Sport Nutr Exerc Metab. 2019;29:152-64.
- [CrossRef] [PubMed] [Google Scholar]






