Translate this page into:
CT-guided steroid injections for the diagnosis and management of piriformis syndrome
*Corresponding author: Ajay Malviya, Department of Orthopaedics, Northumbria Healthcare NHS Foundation Trust, Wansbeck General Hospital, Ashington, United Kingdom. ajay.malviya@newcastle.ac.uk
-
Received: ,
Accepted: ,
How to cite this article: Dharmadhikari R, Pursun Y, Smith C, Verrinder A, Malviya A. CT-guided steroid injections for the diagnosis and management of piriformis syndrome. J Arthrosc Surg Sports Med. 2024;5:65-70. doi: 10.25259/JASSM_23_2024
Abstract
Objectives:
Piriformis syndrome (PS) is an extra-spinal tunnel neuropathy affecting the sciatic nerve. While typically diagnosed clinically, with confirmation through exclusion of other conditions, accurate diagnosis remains challenging. This study assesses the clinical outcomes of computed tomography (CT)-guided corticosteroid injection for suspected PS, considering its potential role as both a diagnostic and a therapeutic intervention.
Materials and Methods:
We conducted a retrospective review of 32 patients suspected to have PS, based on clinical presentation and examination findings, who underwent CT-guided steroid injections between July 2013 and February 2020. Effectiveness was evaluated qualitatively through clinic letters from patient reviews with a mean follow-up of 5 months (range 3–7)
Results:
Thirty-two patients received 44 injections. The cohort had an average age of 45 years, with many being physically active. Initial follow-up showed that 56% experienced pain relief, though 16% of these cases were short-lived. About 19% reported partial benefit, while 31% reported no improvement. Repeat injections were performed on nine patients, with some undergoing up to two repeat procedures. About 47% of patients were discharged following injections, repeats, or surgery, while 53% required further specialist input.
Conclusion:
CT-guided corticosteroid injections appear to be a valuable management tool for diagnosis and treatment of PS, particularly when conservative management fails. However, the long-term benefits are inconsistent, highlighting the need for a more standardized treatment pathway. Given the high degree of diagnostic uncertainty and lack of accurate diagnostic tools for PS, we propose the use of local anesthetic and steroid injections as a diagnostic approach, as well as a management option.
Keywords
Piriformis syndrome
CT-guided
Corticosteroids
Pain relief
Diagnostic tool
INTRODUCTION
Background
The piriformis, originating from the anterior aspect of the second, third, and fourth sacral vertebrae, is a small pyramidal-shaped muscle in the gluteal region, which acts as a lateral hip rotator. Piriformis syndrome (PS), deep gluteal pain syndrome, or fat wallet syndrome is an extra-spinal tunnel neuropathy concerning the sciatic nerve, the largest nerve in the body.[1] It exits the pelvis through the greater sciatic foramen inferior to the piriformis muscle and then passes between the greater trochanter and ischial tuberosity along the back of the thigh.[2] The sciatic nerve sits below the piriformis in 80% of the population and runs through it in 17%.[1] It is thought that close contact of these two structures can lead to “sciatica”-type symptoms when the piriformis is hypertrophied, inflamed, or has physiological anatomical variations.[3] Patients with PS typically report consistent symptoms, including pain in the gluteal/buttock region that may “shoot,” burn, or ache down the back of the leg, along with numbness in the buttocks and tingling sensations along the distribution of the sciatic nerve.[1]
PS can broadly be classified into two types: primary and secondary. Primary PS has an anatomical cause, such as a split piriformis muscle, split sciatic nerve, or an anomalous sciatic nerve path. On the other hand, secondary PS occurs as a result of a precipitating cause, which can include macro-trauma, micro-trauma, ischemic mass effect, and local ischemia.[4]
PS is primarily diagnosed clinically.[1] Its diagnosis is challenging due to the lack of validated and standardized diagnostic tests, making it primarily a diagnosis of exclusion.[5] Stretching maneuvers to elicit clinical signs are based around irritating the piriformis muscles and include: Lasègue[6] (straight leg raise), Freiberg[7] (internal rotation of the extended thigh), and Pace[1] (resisted abduction and external rotation of the thigh) signs. Tools such as magnetic resonance imaging (MRI), computed tomography (CT), and electromyography (EMG) used to rule out other conditions such as lumbar radiculopathy, hip pathologies, or spinal stenosis.[1] On most occasions, the radiological investigations and the nerve conduction studies are negative and there is a reliance on targeted injections to confirm the diagnosis.
Prompt diagnosis and treatment is shown to have improved outcomes for patients.[1] Literature recommends initial simple lifestyle modifications to relax the tense piriformis muscle, hence reducing sciatic nerve compression, through stretching exercises, massage, or thermotherapy.[5] Medically, administration of non-steroidal anti-inflammatory drugs or muscle relaxants can be used to provide some relief.[8] International consensus suggests that a failure of conservative management warrants consideration for image-guided local injection of corticosteroid and anesthetic work to improve pain for these patients.[9-11]
Objective
PS is a significant clinical issue, due to challenging diagnosis and substantial impact on quality of life. This necessitates effective diagnostic and therapeutic approaches. The aim of this study is to assess the role of CT-guided corticosteroids injections in the diagnosis and management of PS through a retrospective analysis of prospectively collected data.
MATERIALS AND METHODS
This is a retrospective review of a cohort of patients (n = 32) who received a CT-guided steroid injection performed by a consultant musculoskeletal radiologist (RD). Patients with a diagnosis of PS were referred after failed conservative management. All referrals were made from specialist orthopedic consultants for the injection and these were carried out between July 23, 2013, and February 25, 2020. All patients having undergone the injection in this period were selected. This cohort study did not have a control group.
All patients had presented with deep gluteal pain with clinical findings suggestive of PS, which did not improve after physiotherapy and other means of non-operative treatment. The local diagnostic criteria involve symptoms characterized as deep gluteal pain with radiation down the posterior aspect of thigh, tenderness in the piriformis area and suggestive clinical examination findings, MRI scan, and nerve conduction studies. All patients had a pelvic X-ray and a thorough clinical appointment assessment. All but one had an MRI scan, with three having changes suggestive of piriformis area inflammation. Eighteen patients had nerve conduction studies of which a third had positive findings. The patients who had positive findings on MRI and nerve conduction studies were not the same and 75% of patients in the cohort did not have any positive investigation findings.
The injections were performed in the CT department using aseptic technique with the patients positioned prone on the CT table (Siemens SOMATOM Definition Edge 128 Slice, SIEMENS, Forchheim, Germany). A skin marker was used to mark the area for injection and an 22G spinal needle was used to penetrate the piriformis. Placement was confirmed using CT. A combination of 1 mL of 0.5 levobupivacaine and 40 mg of triamcinolone then injected into the muscle and just superficial to it.
In all cases, it remained a day-case procedure which required no further monitoring. All patients were referred for physiotherapy. Patients were followed up at an average of 5 months (3–7 months) after injection. Effectiveness of the procedure was then measured qualitatively by interpretating clinic letters from the initial patient review. All patients were asked about their activity level, quality of life, complications, extent of pain relief, and duration.
Variables in this study included but were not limited to patient age, pre-injection level of fitness, and variation in post-injection follow-up. Primary outcomes included pain relief, assessed qualitatively during follow-up. Visual analog pain scale results had not been recorded at the time and due to the retrospective nature of this study, clinic letters were interpreted to obtain the data. This is a source of bias, that unfortunately, could not be addressed. Secondary outcomes involved quality of life improvements, procedural complications, and the need for further interventions such as repeat injections or surgery.
RESULTS
Forty-four CT-guided piriformis injections were performed by RD in the study period, in 32 patients. Three out of 32 presented with bilateral symptoms, 14 with unilateral left-sided symptoms, and 15 right-sided.
The average age at the time of the first injection was 48 ± 13 years and all of them were described as fit and well, using no walking aids, and completely independent. Over half of those patients were described as “active” with running being a common exercise. About 12.5% (4 of 32) were described as athletes, with their job relying on this. About 12.5% (4 of 32) were avid runners, who competed in triathlons and running events. This information is illustrated in Figure 1.
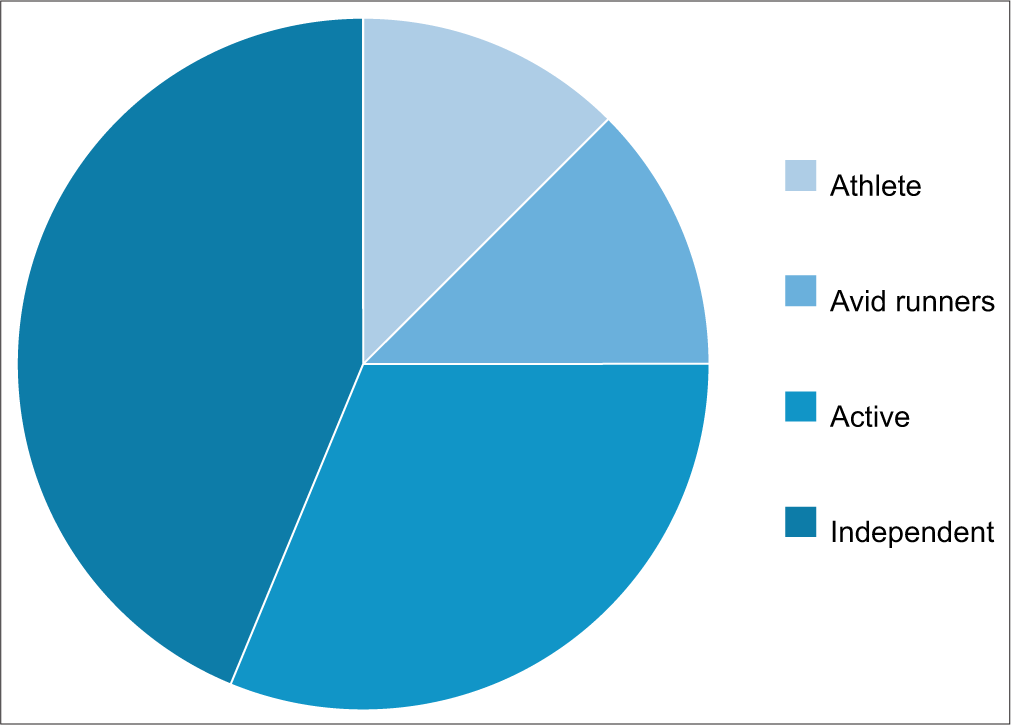
- Cohort fitness levels.
Quality of life assessment was undertaken for the cohort pre-intervention, using qualitative data. Using qualitative data in this study allowed us to capture the nuanced, subjective experiences of patients with PS that quantitative measures alone may miss. It provides descriptive insights that offer a more comprehensive evaluation of patient outcomes.
About 75% (24 of 32) of the sample described a negative effect on their quality of life, especially around exercise, driving, or working. About 50% (16 of 32) of the sample stated that they had reduced exercise tolerance from their normal and 25% (8 of 32) reported pain that disrupted their sleep. This is reported in Figure 2.
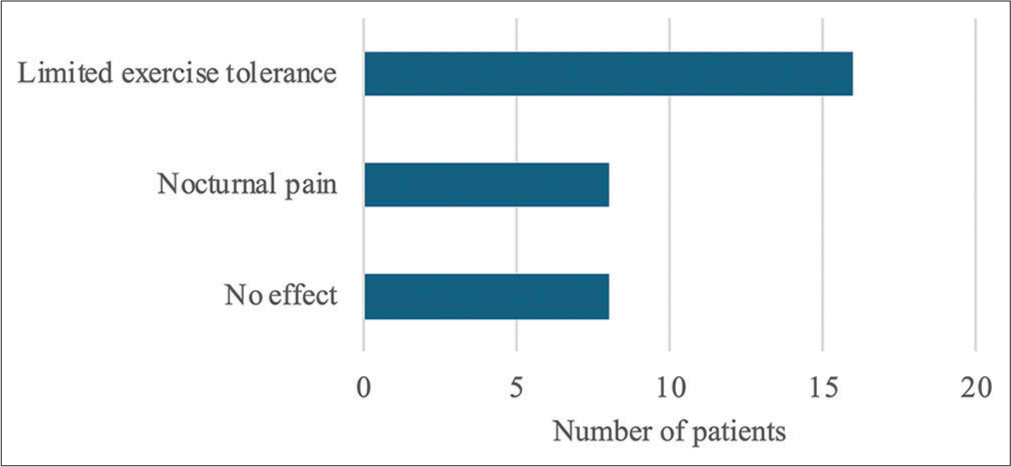
- Quality of life assessment.
While there were no acute complications during any of the injections (infection, bleeding, hematoma, and nerve damage), one patient received, in error, an excessive radiation dose. Instead of a CT-guided injection, a CT hip was performed, and this was managed in line with the local radiation protection rules.
All but one patient received concurrent physiotherapy as per guidelines. At initial follow-up, 56% (18 of 32) had pain relief, although for 16% of these patients, this was short-lived, lasting <3 months. About 19% (6 of 32) of patients had partial benefit and 31% (10 of 32) complained that they experienced no benefit. The rest reported resolution of pain irrespective of the injection or worsening of pain. A comprehensive breakdown of pain relief at follow-up is shown in Figure 3. Patients who did experience pain relief, whether full or partial, were managed using further injections, pro re nata, and eventually discharged from the service. For the other patients, other diagnoses and management options were explored.
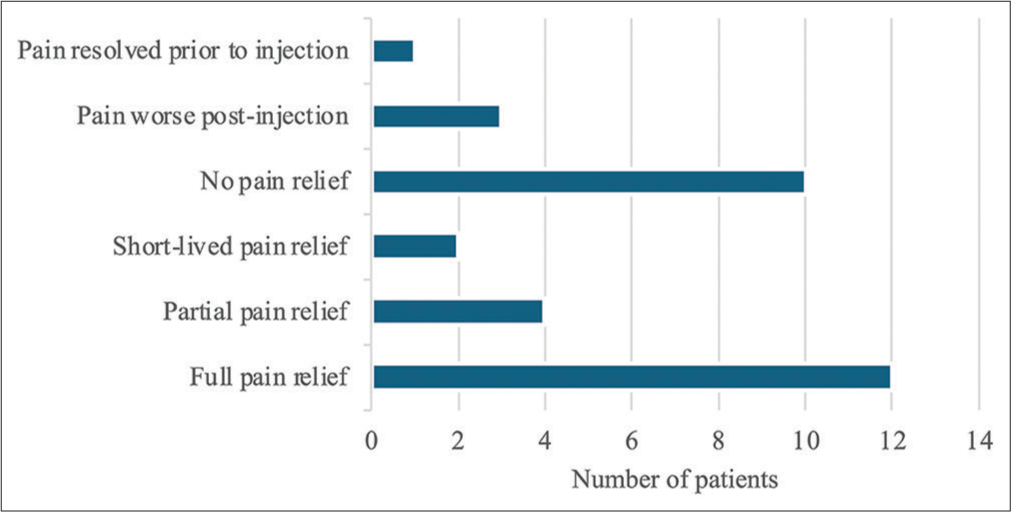
- Pain relief at follow-up.
Nine out of 32 patients had repeat injection; six patients had one repeat and three patients had two repeats, adding up to 12 repeat injections. All the repeat injections were done due to previous good responses to the first injection and were also done for patients with bilateral symptoms, as a way to involve the contralateral piriformis tendon. Average time to repeat the injection was 10 months. Four patients with recurring pain went on to have piriformis-release surgery as definitive management with a 75% success rate.
No significant statistical analysis could be carried out due to relatively simple data as well as small cohort size, but a flow diagram was used to describe each stage of the study. This is shown in Figure 4.
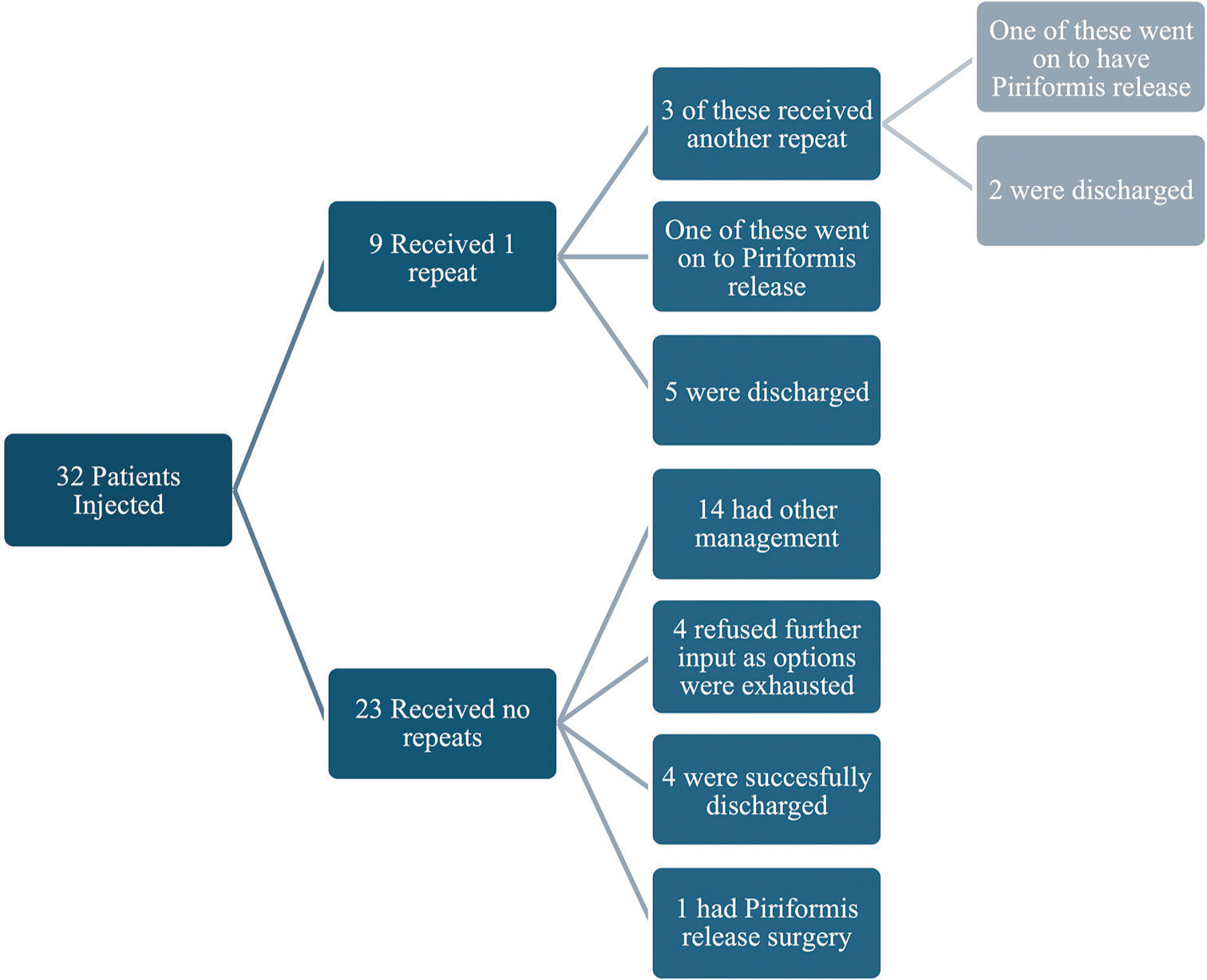
- Summary of all patients included in the study.
DISCUSSION
Deep gluteal pain is a challenging presentation and, therefore, needs a specialist review to ensure the right diagnosis which is made. It can be secondary to several pathologies in the buttock area, with PS being one of the differential diagnoses.[12] The diagnosis is typically made clinically, which has been proved to be a more accurate predictor of treatment success than investigations such as MRI and EMG.[13]
MRI is largely used to exclude other causes of deep gluteal pain and might show an enlarged piriformis muscle as a cause for PS.[14] A brief literature review did not yield a figure for diagnostic accuracy of the pelvic MRI scan for PS. However, only 10% of our cohort had positive findings on MRI. MRI diagnostic accuracy for PS can be upgraded using more varied imaging modalities like magnetic resonance neurography (MRN), which display better structural resolution and improve the contrast to allow better visualization of nerve lesions.[15]
There was no correlation between our patients who had findings on MRI and those with findings on nerve conduction studies, which reflects the literature on investigations for PS.[14]
From our cohort data, diagnostic accuracy was slightly higher for EMG studies, at 33%. The use of EMG in PS diagnosis is centered around the using the peroneal H-reflex, which decreases when the hip is stressed in an adduction-internal rotation position.[16] Clinical diagnostic tools, using stretch tests to elicit pain, have been extensively studied, and they show high sensitivity, specificity, and diagnostic odds ratio.[17,18]
In this study, all patients underwent multiple, multi-modal, and sometimes multi-specialty investigations to make the diagnosis. Most of the patients had gone through long diagnostic journey before being referred for CT-guided injections. Seven patients, or 22% of the sample, had been referred from the spinal orthopedic team, after having undergone numerous investigations and procedures for their presumed sciatica. Despite the patient group receiving numerous investigations, 75% of the samples were treated without any positive test results. Clinical symptoms and signs were instead relied on as diagnostic tool. Moreover, of those that had positive findings on investigations, barely over half had pain relief at three months, reflecting the limited accuracy of these tests. This highlights the less often stated use of injections as a potential diagnostic tool.
The CT-guided piriformis injection can, therefore, successfully be used as an effective investigation tool, on the background of negative imaging findings. Our study demonstrates the benefit and superiority of diagnostic injections in the management of deep gluteal pain. We have found it to be a relatively safe procedure, with low complication rates, comparable to MRI scans or EMG.[19] We recognize that our exploration of the diagnostic value of injections in PS is preliminary and requires further investigation. Our study supports the clinical application of these injections for diagnostic purposes, highlighting the necessity for additional research to validate and explore their broader implications.
The use of corticosteroid injections as a diagnostic tool in orthopedics is widely documented. Protheroe and Gadgil,[20] reported statistically significant data supporting methylprednisolone injections as a viable diagnostic and therapeutic option for mid-foot osteoarthritis. A meta-analysis done by Najm et al.,[21] showed short-term reduced pain and improved function following glucocorticoid injection in the knee, hence confirming an osteoarthritis diagnosis. While it is acknowledged that these are intraarticular injections, we are proposing local anesthetic and corticosteroid injection as a standardized diagnostic and therapeutic tool for PS, given the lack of existing accurate assessment modalities.
As a therapeutic tool, and on reviewing patient outcomes over time, the picture of success is less clear. Of the 75% who had some pain relief at initial follow-up, only 29% of these were successfully discharged within a year, the rest of them having other associated pathologies for which they have been under the care of other clinicians. Overall, 47% of the sample were discharged following the original piriformis injection, repeats, or surgery, but 20% of these cases was due to the patient accepting the pain and wishing to have no further input. This figure represents a common “real-life” clinical scenario where patients elect to manage their condition without further intervention and while this may initially appear to diminish the therapeutic value of the injection, they highlight the necessity for individualized patient care with shared decision-making and the challenges in standardizing treatment outcomes for PS. The other 53% of patients went on to have further input from other orthopedic specialists such as the spinal team, or hip specialists for either the joints or muscles in this area. This underscores the complexity of treating PS with some patients presenting with associated pathologies and the variability in individual responses to treatment.
The literature supports that CT-guided piriformis injections are a successful, relatively non-invasive and safe treatment for deep gluteal pain, alongside physiotherapy.[10] Novel research is being carried out globally, such as the investigating the use of ultrasound-guided piriformis injections to provide the same accuracy and efficacy without the radiation risk.[22] However, the diagnosis and management of PS is still unclear.[12] Pain from PS has not been adequately studied and further studies using a standardized protocol to look quantitatively at pain before injection and at regular time intervals following the injection would be an appropriate next step. Pain is inherently a subjective and personal experience, and it is a challenge to compare this reliably, especially without before and after quantitative measurements.[23]
This study revealed that the pathway for repeat injections and further invasive management seems relatively ad hoc. Added standardization could help create a reliable, robust pathway for patients, especially as current evidence suggests surgery should only be considered as a last resort. Work is being done internationally with different steroid dosing and Botox CT-guided piriformis injections.[24] A multi-trust or regional study would help us increase sample size, compare referral pathway, dose regimen, and follow-up protocols, to increase the accuracy of recommendations so as to improve patient outcomes.
Limitations
This study’s primary limitation was its retrospective nature. The effectiveness was measured only qualitatively, and standardized clinical outcome scores were not recorded. It was outside the scope of this paper to comment on quantitative data because this was not recorded. However, future studies on the subject are necessary and should pre-emptively record clinical outcome scores and visual analog scales for pain. It is also challenging, with diagnostic uncertainty and limited follow-up information to understand these findings. We cannot say with certainty whether the injection had no effect or a negative effect for patients; the diagnosis could be wrong, and pain can be multifactorial. The review period after the injection was also very variable, due to waiting time pressures, and this could have affected the patient’s perspective of their recovery. Finally, a small population size rendered relevant statistical analysis unreliable.
CONCLUSION
This study demonstrates that CT-guided corticosteroid injections can serve as both a diagnostic and therapeutic tool for PS, particularly in patients who have not responded to conservative treatments. Despite the variability in diagnostic accuracy from other modalities such as MRI and EMG, the clinical response to these injections provides valuable insights for diagnosing PS. While our data show that initial pain relief was achieved in 75% of patients, the long-term benefits were less consistent.
The findings suggest that while CT-guided injections are a viable non-invasive treatment option, the pathway for managing PS post-injection remains somewhat unstructured. The variability in follow-up and patient outcomes highlights the need for a more standardized approach to both the diagnostic process and subsequent treatment plans. Implementing a multidisciplinary strategy and exploring further imaging modalities, such as MRN, could improve diagnostic accuracy and patient outcomes.
Future research should focus on larger, multi-center studies to establish standardized protocols for the use of corticosteroid injections in PS. This includes consistent dosing, follow-up intervals, and quantitative pain assessment pre- and post-injection. By refining these protocols and exploring alternative imaging techniques, we can enhance the accuracy of PS diagnoses and improve the efficacy of treatments, ultimately leading to better patient care and outcomes.
Acknowledgment
This study has been presented at a local meeting in Northumbria health-care NHS foundation trust as well as a British Hip Society meeting.
Author’s contributions
AV was responsible for the study concept and design. She was supervised by RD who carried out the injections and whose patients were recruited for the study. CS collated the data succinctly and produced the relevant charts included in this paper. This data was then used by YP to prepare the manuscript, which was then edited and reviewed by AM. AM and RD take responsibility for the integrity of this work.
Ethical approval
The Institutional Review Board approval is not required since it was a retrospective review study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Piriformis syndrome In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024.
- [Google Scholar]
- Anatomy, sciatic nerve In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024.
- [Google Scholar]
- The clinical features of the piriformis syndrome: A systematic review. Eur Spine J. 2010;19:2095-109.
- [CrossRef] [PubMed] [Google Scholar]
- The piriformis syndrome-a special indication for botulinum toxin. Nervenarzt. 2020;91:99-106.
- [CrossRef] [PubMed] [Google Scholar]
- Piriformis syndrome, diagnosis and treatment. Muscle Nerve. 2009;40:10-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of piriformis thickness, hip muscle strength, and low back pain patients with and without piriformis syndrome in Malaysia. Life. 2023;13:1208.
- [CrossRef] [PubMed] [Google Scholar]
- The piriformis muscle syndrome: A simple diagnostic maneuver. Neurosurgery. 1994;34:512-4. discussion 514
- [CrossRef] [Google Scholar]
- Confirmation of needle placement within the piriformis muscle of a cadaveric specimen using anatomic landmarks and fluoroscopic guidance. Pain Physician. 2008;11:327-31.
- [CrossRef] [PubMed] [Google Scholar]
- Computed tomography-guided percutaneous infiltrations for piriformis syndrome: A single-center retrospective study. J Long Term Effect Med Implants. 2020;30:113-8.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided piriformis muscle injection for the treatment of piriformis syndrome. Turk Neurosurg. 2014;24:471-7.
- [CrossRef] [PubMed] [Google Scholar]
- Piriformis syndrome: Long-term follow-up in patients treated with percutaneous injection of anesthetic and corticosteroid under CT guidance. Cardiovasc Intervent Radiol. 2012;35:375-82.
- [CrossRef] [PubMed] [Google Scholar]
- Brief review: Piriformis syndrome: Etiology, diagnosis, and management. Can J Anesth. 2013;60:1003-12.
- [CrossRef] [PubMed] [Google Scholar]
- Four symptoms define the piriformis syndrome: An updated systematic review of its clinical features. Eur J Orthop Surg Traumatol. 2018;28:155-64.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of magnetic resonance neurography for diagnosis of piriformis muscle syndrome and verification of the effect after botulinum toxin type A injection. Medicine. 2015;94:e1504.
- [CrossRef] [PubMed] [Google Scholar]
- Peroneal H-reflex in piriformis syndrome (P07.057) Neurology. 2013;80(7 Suppl):07.057.
- [CrossRef] [Google Scholar]
- Piriformis syndrome: Diagnosis, treatment, and outcome-a 10-year study. Arch Phys Med Rehabil. 2002;83:295-301.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region. Knee Surg Sports Traumatol Arthrosc. 2014;22:882-8.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided injection of botulinic toxin for percutaneous therapy of piriformis muscle syndrome with preliminary MRI results about denervative process. Eur Radiol. 2001;11:2543-8.
- [CrossRef] [PubMed] [Google Scholar]
- Guided intra-articular corticosteroid injections in the midfoot. Foot Ankle Int. 2018;39:1001-4.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of intraarticular corticosteroid injections in knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Joint Bone Spine. 2021;88:105198.
- [CrossRef] [PubMed] [Google Scholar]
- Computer-tomographic verification of ultrasound-guided piriformis muscle injection: A feasibility study. Pain Physician. 2014;17:507-13.
- [CrossRef] [PubMed] [Google Scholar]
- Orthopedic professionals' recognition and knowledge of pain and perceived barriers to optimal pain management at five hospitals. Healthcare. 2018;6:98.
- [CrossRef] [PubMed] [Google Scholar]
- Piriformis syndrome: Pain response outcomes following CT-guided injection and incremental value of botulinum toxin injection. Diagn Interv Radiol. 2021;27:126-33.
- [CrossRef] [PubMed] [Google Scholar]






