Translate this page into:
Articular cartilage regeneration: A current concepts review
*Corresponding author: Abhishek Vaish, Department of Orthopedics, Indraprastha Apollo Hospitals, New Delhi, India. drabhishekvaish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vaish A, Vaishya R. Articular cartilage regeneration: A current concepts review. J Arthrosc Surg Sports Med. 2024;5:90-5. doi: 10.25259/JASSM_20_2024
Abstract
Articular cartilage injuries and defects have limited or no repair capacity. Most of the current surgical techniques can produce only fibrocartilage and not the actual hyaline cartilage. This review explores current trends in articular cartilage treatment, focusing on established approaches, emerging therapies, and future directions. A detailed literature search was performed on PubMed, Scopus, Embase, and Google Scholar in May 2024. All the relevant studies were identified and included in this review. While surgical techniques are crucial, non-operative approaches such as physical therapy with targeted mechanical stimulation or pulsed electromagnetic fields, the use of biomarkers for early diagnosis and treatment, and monitoring play a key role in managing symptoms and supporting the regeneration process. Over the past few decades, various surgical techniques have been developed for treating articular cartilage defects more effectively. Despite the field of cartilage regeneration making significant strides, there are still several key research gaps that need to be addressed. The future of cartilage regeneration is brimming with exciting possibilities such as bioprinting, bioengineering, stem cell therapies, gene editing, and the use of artificial intelligence. Many promising techniques show success in pre-clinical studies but translating them into effective and safe clinical treatments requires further research and large-scale clinical trials. Careful consideration of the ethical implications of using these therapies remains paramount. Hence, cartilage regeneration research is a field brimming with potential. While challenges remain, such as optimizing cell delivery and ensuring the long-term functionality of regenerated tissue, the future looks promising.
Keywords
Articular cartilage
Knee
Joints
Stem cells
Genetics
INTRODUCTION
Articular cartilage defects are a significant clinical challenge, leading to pain, stiffness, and functional limitations.[1] Cartilage, unlike most tissues, lacks blood vessels and nerves, hindering its natural repair mechanisms.[2] Current treatments often focus on stimulating the growth of fibrocartilage, a less durable substitute for the original hyaline cartilage. Articular cartilage plays a crucial role in facilitating smooth, near-frictionless movement.[3,4] Defects caused by trauma, osteoarthritis (OA), or other etiologies can lead to pain, stiffness, joint instability, and ultimately, functional limitations. Restoring a functional articular surface is a significant clinical challenge for orthopedic surgeons. While established surgical techniques have offered some success, the field of cartilage repair is rapidly evolving.
This review explores current trends in articular cartilage treatment, focusing on established approaches, emerging therapies, and future directions. We discuss the advantages and limitations of microfracture, autologous chondrocyte implantation (ACI), osteochondral autograft transfer system (OATS), and allograft transplantation.[5,6] We then delve into promising advancements such as stem cell therapy, biomaterial scaffolds, and gene therapy, analyzing their potential for cartilage regeneration.[7,8] Finally, we considered the future of the field, including personalized medicine and the integration of biological and biomechanical strategies.[9]
MATERIALS AND METHODS
A literature search was conducted using PubMed, Scopus, Embase, and Google Scholar on 7th May 2024 using the keywords “Cartilage,” “Chondral,” and “Joint preservation”. Boolean operators used were [AND], [OR]. Only full-text available articles and English language articles were included in the study. Animal studies, in vitro studies, duplicate publications, case reports, and editorials were excluded from the study. The remaining articles were identified and critically analyzed in this review.
RESULTS AND DISCUSSION
Biomarkers
Identifying reliable biomarkers of cartilage health and regeneration is crucial for early diagnosis, treatment monitoring, and assessing the effectiveness of new therapies. The extracellular matrix (ECM) of cartilage undergoes a series of changes at the molecular level during OA progression. These molecular changes can be promising biomarkers, such as cartilage oligomeric matrix protein and thrombospondin-4, which are sensitive enough to be detected at the early stage and help monitor OA progression. Synovial fluid evaluation for detection of biomarkers, which could either be anabolic or catabolic, can help predict the development or progression of OA at an early stage. Many studies have been conducted and testing of more than eighty biomarkers. However, there is no clear conclusion on their significance as results vary among the studies.[10]
Treatment options
We have identified three main groups of treatment modalities for articular cartilage regeneration [Table 1] and these are discussed ahead.
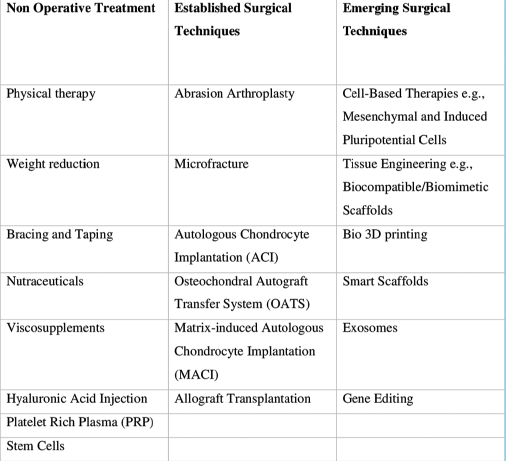 |
Non-operative treatment options
While surgical techniques are crucial, non-operative approaches like physical therapy with targeted mechanical stimulation or pulsed electromagnetic fields and the use of biomarkers for early diagnosis and treatment monitoring, play a key role in managing symptoms and supporting the regeneration process.
Physical therapy
Strengthening exercises for the muscles around the joint help improve stability and reduce stress on the cartilage.[11,12] Range-of-motion exercises help to maintain joint flexibility and function. Advanced physical therapy techniques using targeted mechanical stimulation or pulsed electromagnetic fields are being explored to promote cartilage healing and regeneration.[13,14] Exercise upregulates bone marrow mesenchymal stem cells (BM-MSC) and bone marrow hematopoietic stem cells recruitment, enhances BM-MSC osteogenic, chondrogenic, and apoptotic gene expression, and upregulates BM-MSC expression of osteogenic mitochondrial RNA and the secretion of growth factors (GFs). According to Sumanasinghe et al., bone morphogenic protein-2 (BMP-2) messenger RNA expression and BMP-2 production were upregulated as compared to controls, indicating that mechanical strain associated with exercise can induce osteogenic differentiation of human BM-MSCs.[15]
Weight management
Maintaining a healthy weight reduces stress on the joints, which can slow down cartilage degeneration and alleviate pain.[16,17] As per Sun et al.,[18] weight loss of 20% in patients with obesity and OA by bariatric gastric surgery led to an improvement in pain and physical function and attenuation in systemic inflammation resulting in a structural improvement of cartilage. There is a significant reduction in inflammatory markers such as interleukins (ILs) and cytokines, which directly affect the function of chondrocytes. Hence, there is ample literature available that links weight loss with improved cartilage function as well as healing.
Bracing and taping
Wearing a brace or using Kinesio taping can provide support and stability to the joint, reducing pain and inflammation. These tapes are elastic, waterproof, and can stretch up to 130–140% of its length. The exact mechanism is unknown. However, it works by improving the blood and lymphatic flow, pain reduction by stimulation of mechanoreceptors in the skin, and increasing afferent feedback to the central nervous system.[19]
Dietary supplements
Certain dietary supplements (nutraceuticals), such as glucosamine and chondroitin sulfate, curcumin, and collagen peptide, have shown promise in reducing pain and inflammation associated with OA. The possible mechanisms of action of glucosamine include its preferential incorporation by chondrocytes into the components of glycosaminoglycan chains in the intact cartilage, stimulation of the synthesis of physiological proteoglycans, and decrease the activity of catabolic enzymes, including metalloproteases. It is involved in the suppression of IL-1-induced inflammatory reaction by decreasing nuclear factor- kappa B activation and suppression of superoxide radical generation. However, evidence for their effectiveness in promoting cartilage regeneration is limited.[20,21]
Viscosupplementation
Injections of hyaluronic acid (HA) may provide temporary pain relief in some cases. HA is a naturally existing component of the joint. Injecting a synthetic gel has multiple benefits, such as the promotion of cell adhesion, proliferation, and chondrogenesis potential. It regulates the normal microenvironment of the joint as well as promotes extracellular matrix deposition and repair. There are various commercially available HA-based hydrogels, including alkenyl, aldehyde, thiolate, phenolized, hydrazide, and host– guest group-modified hydrogels.[22,23]
Platelet-rich plasma (PRP)
PRP injections involve injecting concentrated platelets derived from the patient’s blood into the damaged area. PRP can help repair cartilage defects, improve joint function, and reduce OA symptoms. PRP can also affect inflammation, angiogenesis, cartilage protection, and cellular proliferation and differentiation. The GFs present in platelets may stimulate healing and reduce inflammation, although research on its effectiveness for cartilage regeneration is ongoing secretion of a variety of anabolic factors (transforming growth factor-β1 [TGF-β1], IL-4, IL-10, and IL-13) to stimulate the synthesis of ECM, thereby promoting cartilage repair. There was a significant increase in the cartilage thickness and volume after 8 weeks of evaluation. Furthermore, it has been observed that three PRP injections give far better functional outcomes compared to one injection.[24]
Stem cells
While still under investigation for surgical applications, stem cell injections are being explored as a non-surgical approach for cartilage regeneration. Mesenchymal stem cells (MSCs) facilitate that tissue regeneration is seemingly not a simple process. Increasing evidence indicates that the life span of injected/implanted MSCs at the damaged sites was much shorter than expected. The bioactive paracrine factors secreted by MSCs play some if not all, beneficial effects in modulating the microenvironment of the damaged tissue, leading to more favorable conditions for tissue regeneration. The diverse biological functions include immune regulation, angiogenesis, antiapoptotic, antioxidative, cell homing, and promotion of cell differentiation. MSCs release cytokines to initiate cartilage repair, which is followed by chondrogenic proliferation together with secretion of ECM proteases and GFs such as TGF-β, insulin-like GF-1, and fibroblast GF. More research is needed to determine their long-term safety and efficacy.[25,26]
Established surgical techniques
Over the past few decades, various surgical techniques have been developed for treating articular cartilage defects, and some of the established methods are discussed ahead:
Microfracture and abrasion arthroplasty
This minimally invasive technique involves creating small subchondral microfractures to stimulate bleeding and the formation of fibrocartilage. The quality of cartilage repair following microfracture is variable and inconsistent due to unknown reasons. Younger patients have better clinical outcomes and quality of cartilage repair than older patients. When lesion location was shown to affect microfracture outcome, patients with lesions of the femoral condyle had the best clinical improvements and quality of cartilage repair compared with patients who had lesions in other areas. This technique is simple, and there is no thermal damage to the joint. Patients with smaller lesions have better clinical improvement than patients with larger lesions. Microfracture is a relatively simple procedure suitable for small defects but may not provide long-term durability, particularly in weight-bearing joints.[27]
ACI
Large cartilage defects, kissing lesions, and OA cannot be addressed by microfracture. ACI has been recommended for the treatment of symptomatic cartilage defects of approximately 2.5–10 cm2. ACI is a two-stage procedure, beginning with arthroscopic assessment of the chondral injury and biopsy to harvest approximately 200–300 mg of cartilage, followed by a commercial enzymatic digestion and cell expansion in monolayer culture with cryopreservation of the cells. After 3–6 weeks, cultured cells are received which are implanted into the prepared defect. The first and second-generation techniques involved stitching the periosteal flap and collagen membrane over the defect and injecting the cultured cells under, respectively. Third-generation ACI offers hyaline cartilage repair by impregnating the cultured cells in a hydrogel scaffold. This technique is effective but requires two surgical procedures and may lead to donor site morbidity.[28]
OATS
OATS involves transplanting healthy osteochondral plugs from a non-weight-bearing area of the joint to the defect site. This technique offers a direct replacement of damaged cartilage with healthy tissue but is limited by donor site availability and potential for morbidity. This technique is highly effective in focal cartilage defects. It provides excellent functional outcomes and fast recovery. The cartilage is hyaline in nature. Hence, durability and long-lasting results are assured in this technique.[29]
Matrix-induced autologous chondrocyte implantation (MACI)
This two-step procedure utilizes a patient’s cartilage cells. First, a small healthy cartilage sample is harvested arthroscopically. In a laboratory, these cells are multiplied and grown on a collagen membrane (the matrix). During a second surgery, the cell-seeded membrane is implanted into the defect site, creating a patch for new cartilage growth. MACI offers a more durable repair compared to microfracture, but is more complex and requires longer recovery.[30]
Allograft transplantation
Allografts involve transplanting healthy osteochondral tissue from a deceased donor. Osteochondral allograft transplantation is a viable option for young active patients with large osteochondral defects, in which native cartilage is preferred over arthroplasty. Fresh allografts are harvested within 24 h and stored after treatment at 4°Celsius. The cell viability starts to decline from day 14 to 21. Hence, they should be transplanted as soon as possible, but the ideal time is 15–28 days when 70% of cells are viable. Ten-year survivorship is 70% in the femur, 60% in tibia, and 40% in bipolar defects. While allografts can address larger defects compared to autografts, potential drawbacks include disease transmission risk, immunologic rejection, and limited long-term durability.[31]
Emerging surgical techniques
The field of cartilage regeneration is constantly evolving, with exciting new developments and emerging techniques on the horizon all the time, as discussed ahead.
Cell-based therapies
MSCs from bone marrow or fat tissue are at the forefront of cell-based therapies. When transplanted into the injured area, these cells can differentiate into cartilage cells and produce a new matrix. Induced pluripotent stem cells (iPSCs) offer another potential source for generating large quantities of cartilage cells, although ethical considerations remain.[32]
Tissue engineering
Biocompatible/biomimetic scaffolds combined with GFs and cells hold promise for creating new cartilage implants that mimic the structure and function of natural cartilage. Simply injecting stem cells is not enough. Biocompatible scaffolds provide a supportive structure for cell growth and guide tissue formation. These scaffolds can be seeded with cells and GFs that further stimulate cartilage regeneration. Bioprinting offers a promising avenue for creating personalized scaffolds with precise 3D structures containing a patient’s cells.[33]
Smart scaffolds
Scaffolds that can release GFs or respond to mechanical stimuli are being developed to create a dynamic environment that optimizes cartilage regeneration.[34]
Exosomes
Exosomes are miniscule extracellular vesicles released by cells. Studies suggest that exosomes derived from stem cells can promote cartilage repair by influencing the surrounding tissue. This cell-free approach offers a potentially safer and more manageable therapeutic option.[35]
Gene editing
Gene editing techniques like CRISPR-Cas9 are being investigated to modify stem cells to enhance their chondrogenic potential (ability to form cartilage) and improve regeneration outcomes.[36]
Research gaps
The field of cartilage regeneration is making significant strides, but there are still several key research gaps that need to be addressed [Figure 1]. Optimizing cell delivery methods, ensuring the long-term functionality of regenerated cartilage, and developing cost-effective and widely accessible therapies are key areas of focus. In addition, a deeper understanding of cartilage biology, including signaling pathways and the role of mechanobiology, is needed to refine treatment strategies.[37,38] While MSCs and iPSCs show promise, there is a need to look for the most effective and readily available cell source for large-scale regeneration. In addition, developing efficient and minimally invasive ways to deliver cells to the defect site remains a challenge. Designing scaffolds that perfectly mimic the natural cartilage environment and degrade at the right pace for tissue replacement are an ongoing pursuit. The avascular nature of cartilage hinders nutrient and oxygen transport, and hence, developing scaffolds that promote blood vessel formation within the regenerated tissue are a critical area of research. A deeper understanding of the complex signaling pathways that regulate cartilage development and regeneration is crucial to creating targeted therapies. The role of mechanical loading to the cartilage (mechanobiology) in promoting articular cartilage regeneration is gaining interest. It is to be ensured that the regenerated cartilage, by whichever technique, integrates seamlessly with surrounding tissue and maintains its functionality over time is crucial.
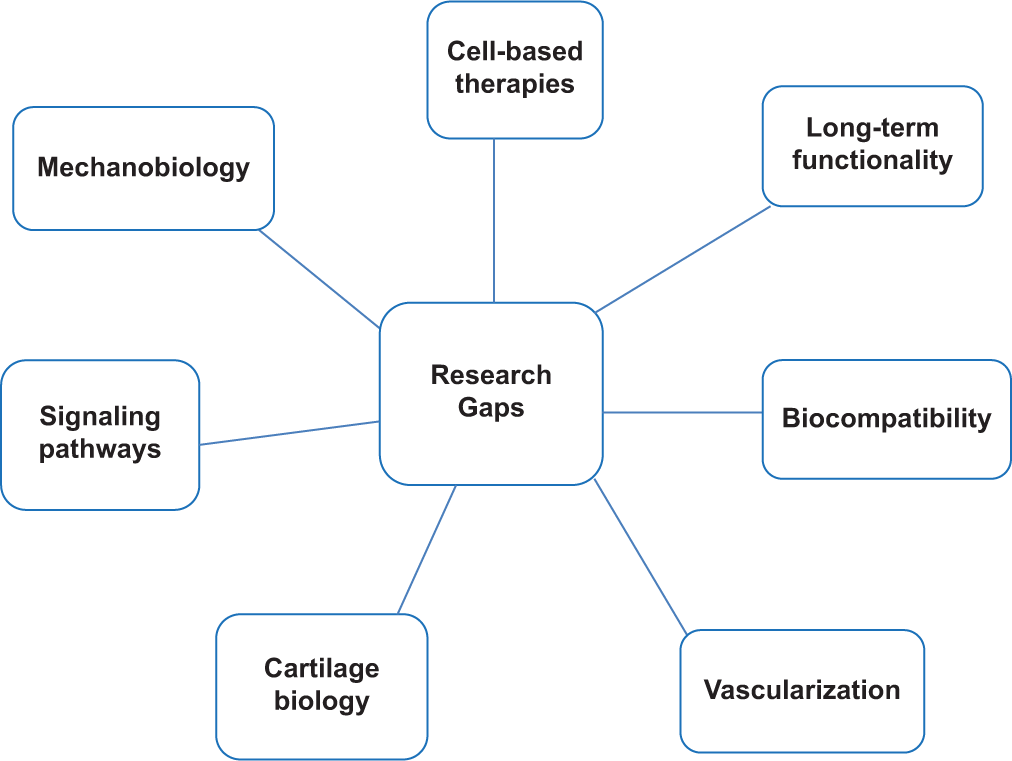
- Research gaps in cartilage regeneration.
By addressing these research gaps, scientists and clinicians can move closer to the goal of achieving true and long-lasting cartilage regeneration, offering patients with joint damage a future free from pain and with restored mobility.
Future directions
The future of cartilage regeneration is brimming with exciting possibilities [Figure 2]. Personalized treatments, with the use of a patient’s cells and bioprinting, hold immense potential. Further advancements in bioengineering, including self-assembling scaffolds and biomaterials that integrate seamlessly with surrounding tissues, are on the horizon. Combination therapies that integrate cell therapies with gene editing or GFs, and the integration of noninvasive monitoring techniques, could lead to comprehensive regeneration protocols. Finally, a focus on prevention through early detection and the development of preventative measures holds promise for a future where cartilage damage is a thing of the past.
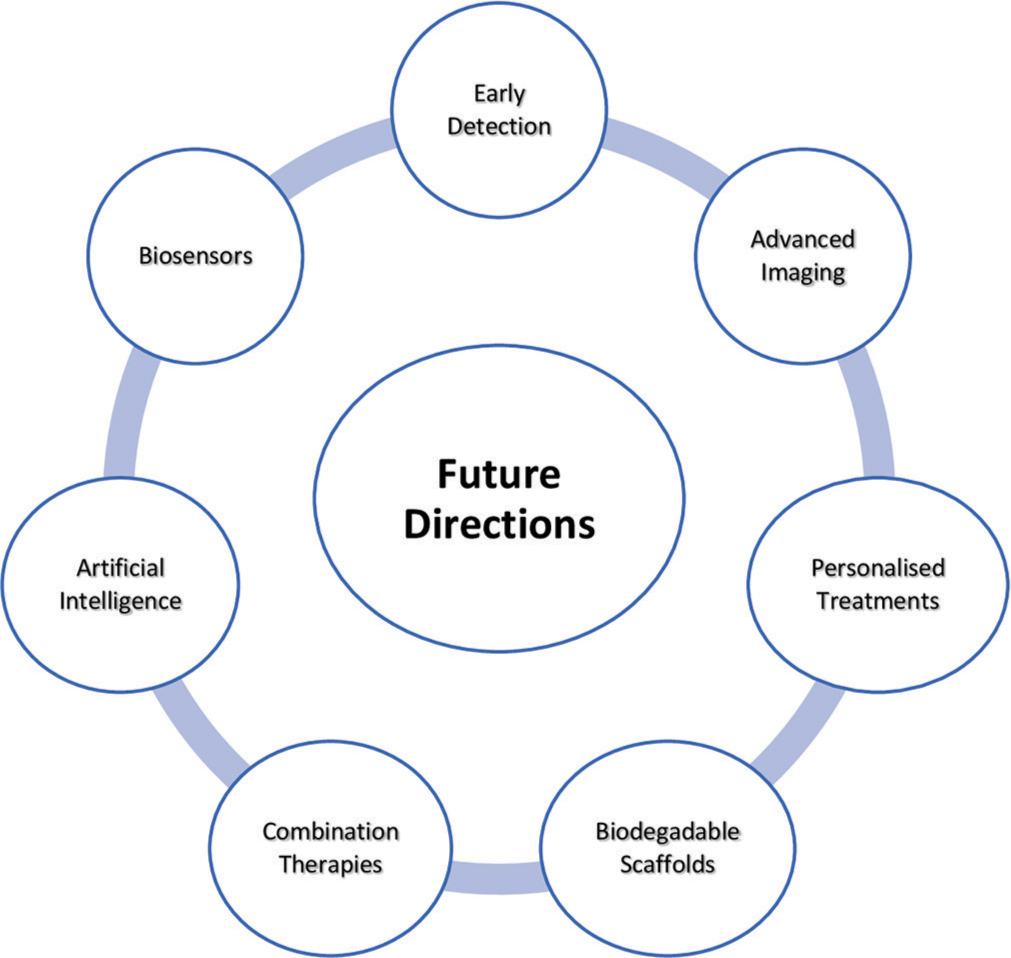
- Future directions in cartilage regeneration.
Integrating physical therapy with biomaterials or advanced modalities like low-intensity shockwave therapy could become a standard for comprehensive regeneration protocols. Artificial intelligence could play a significant role in designing and optimizing biomaterials and scaffolds based on complex biological data. Machine learning algorithms may be used to analyze vast datasets of patient information and treatment outcomes, leading to personalized treatment plans and improved prediction of regeneration success.[39,40]
Advancements in imaging techniques could allow for real-time monitoring of cartilage regeneration progress, eliminating the need for invasive biopsies. Moreover, biosensors implanted within the regenerated tissue could provide valuable data on its health and function, enabling early detection of potential issues.[41]
Challenges and limitations
Cartilage regeneration research is a field brimming with potential. While challenges remain, such as optimizing cell delivery and ensuring the long-term functionality of regenerated tissue, the future looks promising. A deeper understanding of cartilage biology coupled with ongoing advancements in cell therapies, biomaterials, and signaling molecules could lead to the development of regenerative treatments that restore joint health and improve patient quality of life.
Many promising techniques show success in pre-clinical studies, but translating them into effective and safe clinical treatments requires further research and large-scale clinical trials. Furthermore, developing standardized protocols for cell culture, scaffold design, and surgical techniques is crucial for consistent and reliable outcomes. In addition, advanced cartilage regeneration therapies need to be affordable and accessible to a wider patient population.
As gene editing and stem cell therapies become more sophisticated, careful consideration of ethical implications remains paramount. Open discussions and clear guidelines are crucial for the responsible development and application of these technologies.
CONCLUSION
The field of articular cartilage regeneration is experiencing rapid progress and holds immense promise for improving joint health and mobility. However, many of these techniques are still under investigation, and long-term data are needed. In addition, the cost-effectiveness and accessibility of these therapies need to be addressed. By addressing current research gaps and exploring new avenues such as personalized medicine, bioengineering advancements, and preventative strategies, it is becoming a reality to effectively repair and regenerate the cartilage.
Authors’ contributions
Both authors (AV and RV) have been equally involved in conceptualization, literature search, manuscript writing, editing, and final approval.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
Dr Abhishek Vaish and Dr Raju Vaishya are on the editorial board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship
Nil.
References
- Regeneration of articular cartilage defects: Therapeutic strategies and perspectives. J Tissue Eng. 2023;14
- [CrossRef] [PubMed] [Google Scholar]
- The journey of articular cartilage repair. J Clin Orthop Trauma. 2016;7:135-6.
- [CrossRef] [PubMed] [Google Scholar]
- The basic science of articular cartilage: Structure, composition, and function. Sports Health. 2009;1:461-8.
- [CrossRef] [PubMed] [Google Scholar]
- Management of chondral and osteochondral lesions of the hip: A comprehensive review. Orthopadie (Heidelb). 2024;53:23-38.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts of articular cartilage restoration techniques in the knee [published correction appears in Sports Health 2014;6:NP1] Sports Health. 2014;6:265-73.
- [CrossRef] [PubMed] [Google Scholar]
- Non-operative management of osteoarthritis of the knee joint. J Clin Orthop Trauma. 2016;7:170-6.
- [CrossRef] [PubMed] [Google Scholar]
- Biomaterial-based scaffolds in promotion of cartilage regeneration: Recent advances and emerging applications. J Orthop Translat. 2023;41:54-62.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the advantages of 3D bioprinting and 3D spheroids in deciphering the osteoarthritis healing mechanism using human chondrocytes and polarized macrophages. Biomed Mater. 2024;19 10.1088/1748-605X/ad1d18
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning augmented osteoarthritis grading standardization. Tissue Eng Part A 2023 [Last accessed on 2024 June 05]
- [CrossRef] [PubMed] [Google Scholar]
- Cartilage-derived biomarkers in osteoarthritis. Indian J Med Res. 2021;153:41315.
- [CrossRef] [PubMed] [Google Scholar]
- Osteoarthritis and exercise: Does increased activity wear out joints? Perm J. 2000;4:26-8.
- [Google Scholar]
- Qualitative and quantitative evaluation of morpho-metabolic changes in bone cartilage complex of knee joint in osteoarthritis using simultaneous 18F-NaF PET/MRI-a pilot study. Indian J Radiol Imaging. 2023;33:173-82.
- [CrossRef] [PubMed] [Google Scholar]
- Pulsed electromagnetic fields promote repair of focal articular cartilage defects with engineered osteochondral constructs. Biotechnol Bioeng. 2020;117:1584-96.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances and future trends in articular cartilage repair. J Arthrosc Surg Sport Med. 2020;1:159-73.
- [CrossRef] [Google Scholar]
- Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tension strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12:3459-65.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of obesity on medial tibiofemoral cartilage mechanics in females-an exploration using musculoskeletal simulation and probabilistic cartilage failure modelling. Life (Basel). 2023;13:270.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging role of integrated PET-MRI in osteoarthritis. Skeletal Radiol. 2021;50:2349-63.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of diet induced weight reduction on cartilage pathology and inflammatory mediators in the joint tissues. Front Med (Lausanne). 2021;8:628843.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of Kinesio tape and an ankle brace on the lower extremity joint motion in fatigued, unstable ankles during a lateral drop landing. Int J Environ Res Public Health. 2021;18:6081.
- [CrossRef] [PubMed] [Google Scholar]
- Chondroitin for osteoarthritis. Cochrane Database of Syst Rev. 2015;2015:CD005614.
- [CrossRef] [PubMed] [Google Scholar]
- Glucosaminesulfate use and delay of progression of kneeosteoarthritis: A 3-year, randomized, placebo controlled, double-blind study. Arch Intern Med. 2002;162:2113-23.
- [CrossRef] [PubMed] [Google Scholar]
- The role of hyaluronic acid in cartilage boundary lubrication. Cells. 2020;9:1606.
- [CrossRef] [PubMed] [Google Scholar]
- Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering. Mater Today Bio. 2022;17:100495.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma and cartilage repair. Curr Rev Musculoskelet Med. 2018;11:573-82.
- [CrossRef] [PubMed] [Google Scholar]
- The upsurge in research and publication on articular cartilage repair in the last 10 years. Indian J Orthop. 2019;53:586-94.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020;11:2041731420943839.
- [CrossRef] [PubMed] [Google Scholar]
- Microfracture: Its history and experience of the developing surgeon. Cartilage. 2010;1:78-86.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic autogenous osteochondral mosaicplasty. Hungarian J Orthp Trauma. 1996;39:49-54.
- [Google Scholar]
- Use of MACI (autologous cultured chondrocytes on porcine collagen membrane) in the United States: Preliminary experience. Orthop J Sports Med. 2020;8:2325967120941816.
- [CrossRef] [PubMed] [Google Scholar]
- Osteochondral allograft transplantation in cartilage repair: Graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Induced pluripotent stem cells in cartilage repair. World J Orthop. 2016;7:149-55.
- [CrossRef] [PubMed] [Google Scholar]
- Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes (Basel). 2020;10:348.
- [CrossRef] [PubMed] [Google Scholar]
- Smart biomaterials for articular cartilage repair and regeneration. Adv Funct Mater. 2023;33:2212561.
- [CrossRef] [Google Scholar]
- Potential of exosomes as cell-free therapy in articular cartilage regeneration: A review. Int J Nanomedicine. 2021;16:6749-81.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic scissors CRISPR/Cas9 genome editing cutting-edge biocarrier technology for bone and cartilage repair. Bioact Mater. 2022;22:254-73.
- [CrossRef] [PubMed] [Google Scholar]
- Publication trends and knowledge mapping in 3D printing in orthopaedics. J Clin Orthop Trauma. 2018;9:194-201.
- [CrossRef] [PubMed] [Google Scholar]
- A minimally invasive revolution: The future of arthroscopy and sports medicine. J Arthrosc Surg Sports Med. 2023;4:27-9.
- [CrossRef] [Google Scholar]
- AI MSK clinical applications: Cartilage and osteoarthritis. Skeletal Radiol. 2022;51:331-43.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning to predict mesenchymal stem cell efficacy for cartilage repair. PLoS Comput Biol. 2020;16:e1008275.
- [CrossRef] [PubMed] [Google Scholar]
- Mitochondria and mitophagy: Biosensors for cartilage degradation and osteoarthritis. Osteoarthritis Cartilage. 2018;26:989-91.
- [CrossRef] [PubMed] [Google Scholar]






