Translate this page into:
Graft fixation techniques in anterior cruciate ligament reconstruction – A narrative review
*Corresponding author: Easwar Elango, Department of Orthopedics, Gleneagles Health City, Chennai, Tamil Nadu, India. dreaswarortho92@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Elango E. Graft fixation techniques in anterior cruciate ligament reconstruction – A narrative review. J Arthrosc Surg Sports Med. 2024;5:96-106. doi: 10.25259/JASSM_12_2023
Abstract
Anterior cruciate ligament (ACL) reconstruction is one of the most common knee arthroscopic surgeries performed worldwide with 75–90% patients reporting good or excellent outcomes. Implants used for fixing ACL graft during arthroscopic ACL reconstruction are of numerous designs and materials. An implant should be used after going through its biomechanical properties, clinical outcomes, and complications. Rational use of implants according to the graft and patient should be considered. An ideal fixation device should be mechanically adequate enough to hold the graft firmly while biological healing of the graft takes place. This review article summarizes the biomechanical properties, clinical outcomes, and complications of the implants available in the current trend.
Keywords
Anterior cruciate ligament reconstruction
Suspensory fixation
Bioscrews
Cross-pins
Fixed loops
Adjustable loops
INTRODUCTION
Anterior cruciate ligament (ACL) reconstruction is one of the most common knee arthroscopic surgeries performed worldwide with 75–90% patients reporting good or excellent outcomes.[1] The current trend is an anatomical single-bundle reconstruction of ACL using the anteromedial portal for femoral drilling, which gives superior stability and early return to sports. Despite advances in surgical technique and the variety of grafts available, favorable graft healing in tunnels and ligamentization of grafts is required before returning to sports activities.[2,3]
Lutz, in his study of ACL autograft maturation on sequential magnetic resonance imaging (MRI), stated that ACL graft approximates the appearance of native ACL at 1- and 2 years postoperatively.[4] The findings in MRI correspond with the histological maturation of ACL autograft. ACL autograft has to undergo the phase of avascular necrosis, revascularization, resynovialization, and remodeling [Figure 1].[5]
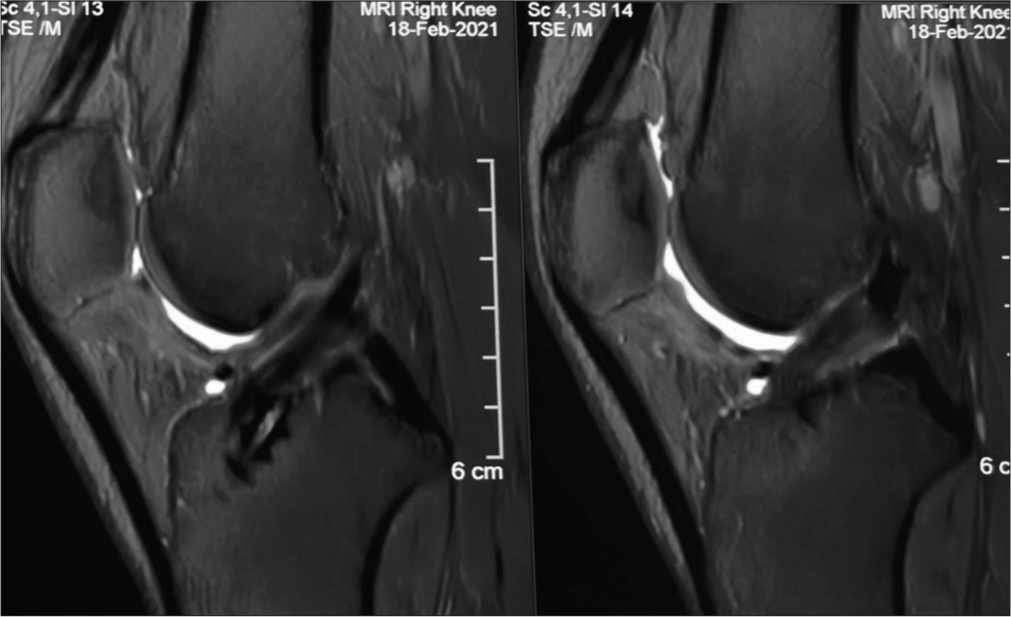
- Magnetic resonance imaging RI T2 sagittal section showing graft looking similar to the native anterior cruciate ligament at one-year follow-up.
Histological and radiological studies showed that revascularization starts as early as three weeks and peaks at six months followed by constant decrease.[2,4] Biochemical analysis of reconstructed ACL done by Marumo et al. showed that total collagen content and non-reducible/reducible collagen crosslinks increase significantly and resemble that of native ACL at the end of one year.[6] Ultrastructural analysis at end of two years showed that cellular and myofibroblast density was almost similar/more than the native ACL.[7] However, collagen fibrils in reconstructed ACL developed unimodality of diameter distribution instead of reaching bimodality. Even though there is partial graft metaplasia at ultrastructural level, there was no decline in function of reconstructed ACL.[8]
Graft healing in bone tunnels is a complex process influenced by type of graft used, method of fixation, tunnel placement, graft motion, and tensioning. In humans, tendon and ligament has two types of insertions. (a) Direct or chondral ligament insertion – Native ACL insertion and patella tendon insertion at bone is characterized by chondral insertion.[9] Ishibashi et al., in a histological study, concluded that this chondral insertion of patella tendon to bone is retained after months of transplantation and appeared to have shifted to the proximal patella tendon-tunnel wall with time.[10] The presence of fibrocartilage in patella tendon recreates the native insertional anatomy of ACL.[6] Incorporation of bone plug into the surrounding host bone completes by 12 weeks which is quite early than healing of soft-tissue grafts. Indirect or fibrous insertion is histologically characterized by three zones-dense connective tissue, woven bone, and lamellar bone.[11] Collagenous Sharpey-like fibers which appear at 12 weeks and mature by 24 weeks anchors the tendon graft penetrating the woven and lamellar bone.[12] This phenomenon is seen with both suspensory fixation and aperture fixation. The presence of these fibers correlates with enhanced interface strength, as all the grafts failed by pullout testing only at the tunnel before 12 weeks and at midsubstance after 12 weeks in a dog study by Rodeo et al.[13] Since biological healing of soft-tissue grafts and bone tendon grafts at intratunnel level takes at least 12 weeks, mechanical fixation of grafts with devices is essential for the early accelerated rehabilitation. The biomechanical properties of the entire graft construct would be the same as native ACL and is partly determined by the method of fixation, and also by the effect of cyclical loading. The ideal fixation device should allow biological healing of the graft, reduces graft motion inside the tunnel, and maintains the adequate graft tension and withstand forces on the graft resulting from accelerated rehabilitation protocols.[14]
Fixation devices in history evolved over time. Broadly graft fixation methods can be classified into indirect or suspensory fixation and direct or aperture fixation. In suspensory mode of fixation, fixation device suspends the graft inside the bone tunnel. Examples include cortical suspensory buttons, screw and washer, cortical plate, and screw. In aperture fixation, graft is compressed against the wall of the bone tunnel. Aperture fixation devices include metal and biodegradable interference screws.[15]
This article reviews our current knowledge on the options available for fixation of ACL graft. Biomechanical properties, clinical outcome, risks, and complications associated with each fixation device will be discussed.
BIOMECHANICS OF ACL GRAFT FIXATION
In vitro biomechanical analysis of ACL graft and ACL graft-fixation construct was necessary to make sure that it can withstand loads from daily activities and accelerated rehabilitation protocol. Single cyclical load to failure analysis of ACL graft and graft fixation construct gives ultimate failure loads in N (Newton). This value represents a catastrophic event such as a fall or traumatic incident.[15] The ultimate failure load for native ACL was found to be 1725– 2160N, 2977 for bone patellar tendon bone graft, 4090N for hamstring tendon preparation, and 2352N for quadriceps tendon grafts. Bone patella tendon was the strongest, with a mean strength of 159–168% of native ACL.[16] These values represent the graft alone and do not include the fixation of the graft to the bone. Forces in the ACL during various movements range from 20N to almost 600N.[17] Forces in ACL while walking were found to be 150N, single leg stance 303N, and jogging 450N.[18,19] Hence, the graft fixation construct should demonstrate an ultimate failure load >590N, which is needed for early rehabilitation after anterior cruciate ligament reconstruction (ACLR).[18] Aggressive activities such as jumping, running, and sports should be avoided until graft-bone healing occurs 12 weeks after ACLR.
Halonen et al.[20] suggest that stiffness is the crucial parameter in ACL reconstruction, and optimal stiffness and prestrain are required to restore the joint motion as well as stresses and strain distribution of the articular cartilage. The graft fixation construct is 4–40 times less stiff than the graft itself. Hence, the fixation method with maximum stiffness should be chosen to match the stiffness of native ACL. The entire stiffness of the reconstruction complex should be close to the native ACL stiffness for controlling the anterior translation of the tibia.[15,20] Hence, stiffness is an important parameter to be considered during the measurement of implant stability. The stiffness of the graft-fixation construct is calculated from the load versus elongation curve as the ratio of the applied load and the corresponding deformation.[21] Most of the studies preferred cyclical loading than single cycle load for evaluation of the stiffness of the construct, as cyclical loading represents the repetitive loading pattern that occurs during post-operative rehabilitation protocol.[15] Forces were applied cyclically, and resulting elongation should be measured at the graft level and graft fixation level. Benca et al.[21] revealed that stiffness calculation using machine displacement would result in a precise, but inaccurate prediction, allowing only for a qualitative comparison between the different fixation methods.
Benca et al.[21] measured relative motion at each end of the graft fixation construct and found that the displacement of the distal graft end was significantly lower than the displacements of the proximal graft end and machine actuator. Many biomechanical studies have defined clinical failure at a 3-mm threshold of machine displacement. However, according to Benca et al.,[21] this corresponds to less than one millimeter of actual graft slippage. Hence, most of the displacement results from graft elongation, stretching of the loops or sutures in the implant rather than graft laxity. Thus, graft migration and graft elongation are different entities. Graft migration or graft slippage is the distal displacement of the graft relative to the tunnel wall, which depends on the strength of the respective fixation technique. Not only migration of the graft, but graft strain also contributes to the graft fixation complex elongation.[22] Thus, graft fixation complex elongation is determined by both the tensile properties of the graft and the strength of the fixation system.[21]
SUSPENSORY FIXATION
Suspensory fixation devices suspend the graft inside the bone tunnel. These devices are capable of maximizing the amount of graft inside the bone tunnel, which helps in graft healing. The devices are broadly classified into extracortical suspensory devices and transcondylar cross-pins. Extracortical suspensory devices include a cortical button with either a fixed loop or an adjustable loop wherein the button rests on the cortex of femur and the loop holds the folded tissue until healing occurs. Fixed loop devices biomechanically and clinically studied includes EndoButton CL (Smith & Nephew, Andover, MA), RetroButton (Arthrex, Naples, FL), and ToggleLoc (Biomet, Warsaw, IN).
Adjustable-loop devices include ToggleLoc with Ziploop (Biomet, Warsaw, IN), ACL TightRope RT I, and ACL TightRope RT II (Arthrex, Naples, FL). In ACL TightRope RT II, there are suture tapes instead of round sutures in TightRope RT I [Figure 2]. The all-inside technique of ACL reconstruction was mentioned in the manuscript which was described by Lubowitz et al.[23] This technique features closed-socket tunnels with less bone removal, dual (femoral and tibial) suspensory fixation, and smaller skin incisions.
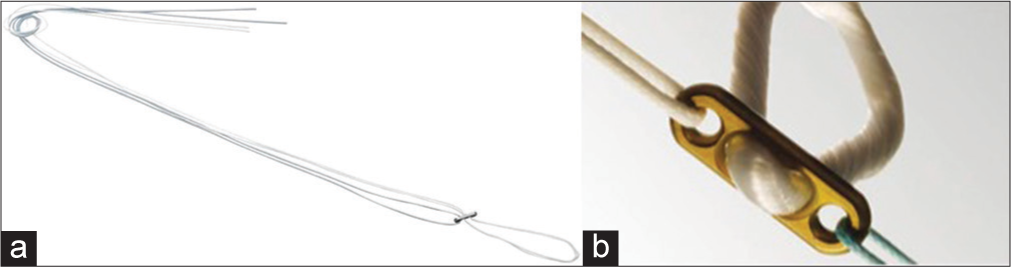
- Extracortical suspensory fixation (a) adjustable loop (Arthrex-TightRope RT). (b) Fixed loop (Smith & Nephew EndoButton).
Cross-pins available are broadly categorized into corticocancellous suspensory device (TransFix and BioTransFix – Arthrex) and cancellous suspensory device (RigidFix – DePuy, Mitek). Hakimi et al. showed that in UK, for femoral fixation of soft-tissue grafts, 79% were using suspensory mode of fixation wherein EndoButton was most commonly.[24] Even though these devices have the strongest ultimate failure loads and stiffness, the most common concern of these devices are displacement on cyclical loads and intratunnel graft motion leading to tunnel widening.
Tunnel widening
Saccomanno et al.[25] mentioned that there was significantly more femoral tunnel widening with the use of the EndoButton compared to cross-pins for fixation within the femoral tunnel. Intratunnel graft motion leading to tunnel widening is divided into longitudinal motion (bungee effect) and transverse motion (windshield wiper effect).[25] Hoher et al. proved that when the distance between tendon tissue and button increased, graft tunnel motion also increased.[26] This explained the finding of Fauno et al. that both femoral and tibial tunnel widening was greater with EndoButton where the fixation point away from the joint. Hence, not only intratunnel graft motion leads to tunnel widening, but also, non-anatomical tunnel placement[27] and aggressive rehabilitation[28] are other mechanical causes for tunnel widening. Silva et al.[29] mentioned that tunnel widening is not only attributed to mechanical causes, but there are also biological causes which include bone resorption due to osteoclast activation by mediators between tunnel and graft, non-specific inflammatory response, and heat necrosis due to drilling.
Similar tunnel enlargement was seen between aperture fixation and extracortical fixation.[30] Therefore, the type of fixation appears not to have a significant effect on tunnel enlargement, since enlargement occurs irrespective of fixation type, suggesting that the enlargement may be more of a biological than a mechanical phenomenon.
Biomechanics
Milano et al.,[31] in his in vitro biomechanical study on porcine knees for femoral fixation of soft-tissue grafts, concluded that ultimate failure load and stiffness are higher for EndoButton than interference screws. The ultimate failure load of EndoButton for soft-tissue femoral fixation was comparatively higher than cross-pins and bioscrews in all biomechanical studies[31,32] with values more than 590N [Table 1] which is the amount of force required for all daily day-to-day to activities. Soft-tissue grafts fixed with EndoButton on the femoral side showed maximum graft-bone displacement after cyclical loading in an in vitro biomechanical study.[31,33] However, graft slippage (i.e., displacement at the distal graft end) was greater for the Rigidfix and interference screws than EndoButton and BioTransFix.[34,35] Hence, corticocancellous devices (Rigidfix) were found to be superior for soft-tissue graft femoral fixation biomechanically in terms of graft elongation, stiffness, ultimate failure load, and extracortical suspensory devices showing variable biomechanical behavior.[31] Interference screws and cancellous suspension devices showed inferior biomechanics for soft-tissue graft femoral fixation. Since cross-pins had associated risks of intra-articular damage, reported cases of supracondylar fracture,[36] pin dislocation, and iliotibial band (ITB) friction syndrome[37] that most of the surgeons prefer to use extracortical suspensory fixation devices than cross-pins for soft-tissue graft femoral fixation.
| Author | Mode of testing and test protocol | Specimen | Femoral fixation implants | Ultimate failure Load (N) | Stiffness (N/mm) | Graft elongation |
|---|---|---|---|---|---|---|
| Ettinger et al.[38] | Cyclic loading of 500 cycles between 60 N and 250 N of 1 Hz | Porcine femora with human tendon grafts | Bioscrews Arthrex |
497.8 | 71.6 | 3.9 mm |
| Bioscrew-Mitek | 543.9 | 48.4 | 4.6 mm | |||
| Staerke et al.[22] | Cyclic loading of 800 cycles between 50 N and 250 N at 1 Hz | Human BTB graft fixed with porcine femur | BTB graft with 7*23 bioscrew | - | - | 0.34 mm |
| Porcine extensor tendons over porcine femur | 50 mm bioabsorbable cross-pin (Bio-TransFix, Arthrex Inc.; Naples/FL, USA) | - | - | 0.67 mm | ||
| 5 mm continuous loop EndoButton (Smith and Nephew Endoscopy; Andover/MA, USA) | - | - | 1.96 mm | |||
| Interference screw | 539 | - | - | |||
| EndoButton | 864 | - | - | |||
| Interference screw | - | - | 5.44 mm | |||
| EndoButton | - | - | 1.75 mm | |||
| Bioscrew | 445N | - | 6–8 mm | |||
| Kamelger et al.[39] | Cyclic loading between 50 N and 250 N at 1 Hz for 1000 cycles. | Porcine femur with only fixation device | EndoButton CL (20 mm loop) | 1024 | 214.7 | 3.6 mm (displacement to failure) |
| RetroButton (20 mm loop) | 798 | 331.5 | 3.2 mm | |||
| ToggleLoc (20 mm loop) | 968 | 232 | 2.6 mm | |||
| ToggleLoc with zip loop technology (adjustable loop) | 876 | 305 | 6.52 mm | |||
| Eguchi et al.[40] | Preload and 2000 cycles | Specimen testing with porcine femur and bovine flexor tendons | EndoButton | 1115 | - | 5.88 mm |
| TightRope RT | 880 | - | 7.74 mm | |||
| ToggleLoc with ziploop | 1334 | 3.34 mm | ||||
| TightRope RT | 859 | 2.74 mm | ||||
| ToggleLoc with ziploop | 830 | 172 | 8.4 mm | |||
| Tape locking screw | 640 | 178 | 5.3 mm |
N: Newton, Hz: Hertz, BTB: bone-tendon-bone, CL: continuous loop, RT: Reverse Tensioning
Fixed loop versus adjustable loop
At present, there are two extracortical suspensory fixation devices in practice: A cortical button with a fixed loop and an adjustable loop. Kamelger et al. did a biomechanical analysis of isolated device testing between various fixed loops (EndoButton, ToggleLoc, and RetroButton) using porcine femurs. All fixed loop implants demonstrated an ultimate failure load of more than 590N, and this improved implant design limits graft tunnel motion. The stiffness of graft–fixed-loop construct reapproximated the native ACL stiffness. Most of the failures in his study occurred due to the breakage of continuous suture loops in these devices.[39]
Fixed-loop devices limit graft migration, but the requirement to drill tunnels for a specific length raises concern in terms of bone preservation and tendon-bone healing in terms of inadequate graft length. However, adjustable-loop devices allow the surgeon to adapt to different tunnel lengths intraoperatively and maximize the amount of graft within the tunnel. Furthermore, adjustable-loop devices have the ability to retension the graft on the femoral side after tibial fixation.[40] Biomechanical analysis of adjustable devices was necessary and should be comparable with the fixed-loop devices for surgical usage. Biomechanical analysis of all adjustable loops, as mentioned in Table 2, demonstrated an ultimate failure load of more than 590N, which corresponds to the commonly accepted recommendation, and also, it was comparable to the fixed-loop devices.[32] Most of the failure occurred by tendon rupture in fixed loops, loop breakage, and slippage in adjustable loops. Stiffness also reapproximated the stiffness of native ACL constructs. Barrow et al.[41] showed significant loop lengthening of TightRope RT device more than 3 mm after fewer cycles (1349 ± 316) than the ToggleLoc (2576 ± 73). With the free suture ends tied, after 4500 cycles, the TightRope had a significant decrease in lengthening. Hence, adjustable-loop devices need to be retensioned after cycling the knee and free suture ends should be tied, which had been shown to decrease cyclic displacement making it biomechanically better. However, fixed-loop devices are always superior than the adjustable-loop devices biomechanically because they allow less cyclic and initial displacement, thus providing better graft femoral fixation in terms of limiting graft slippage and providing sufficient graft strength.
| Author | Mode of testing and test protocol | Specimen | Tibial fixation implants | Ultimate failure load (N) | Stiffness (N/mm) | Graft elongation |
|---|---|---|---|---|---|---|
| Kousa et al.[42] | Cyclic testing-1500 cycles | Porcine tibia | WasherLoc | 975 | ||
| Bioscrew | 612N | |||||
| Intrafix | 1332 | |||||
| Soft silk | 471 | |||||
| Mayr et al.[43] | Cyclic testing | Calf tibia | Adjustable loop | 908 | 6.03 mm | |
| Tibia screw | 693 | 3.33 mm | ||||
| Bioscrew | 476 | 63.9 | 7.564 mm |
Mayr et al., in his biomechanical study, found that tibia suspensory fixation yielded a higher ultimate failure load but with a higher graft elongation.[43] There are no biomechanical studies involving both femoral and tibial suspensory fixation which is used in all inside ACL reconstruction. However, there are clinical outcome comparison studies that exist between all inside ACL reconstruction and standard ACL reconstruction.
Clinical outcome
Saccomanno had done a systematic review of five studies involving 317 patients with a mean follow-up period of 21.7 ± 7.0 months.[25] The Lysholm score, Tegner activity score, and International Knee Documentation Committee (IKDC) score were compiled. The included studies did not report any significant differences in clinical outcomes between extracortical suspensory fixation and transfemoral fixation.[25] Ma et al.[44] showed that rigid aperture fixation using a biodegradable screw and suspensory fixation using EndoButton did not lead to significant differences in clinical outcome at 24–40 months follow-up evaluation. Significant tunnel enlargement was present in both groups, more pronounced on the femoral side. Harilainen et al done prospective randomized clinical comparison of femoral transfixation versus bioscrew fixation in hamstring tendon ACL reconstruction. There was no significant difference in knee laxity, IKDC score, Tegner Lysholm Score.[45]
There are few clinical studies that have compared adjustable-loop and fixed-loop femoral cortical suspension devices, which showed no significant differences in knee stability and the graft failure rate between adjustable- and fixed-loop femoral cortical suspension devices at two years postoperatively in a consecutive series of 188 patients who had undergone primary ACLR using hamstring autograft. However, Choi et al.,[46] in his prospective study, found that femoral fixation by the use of the fixed-loop device resulted in significantly better knee stability on the pivot-shift test than the adjustable-loop device after ACLR with a hamstring graft. However, the patients in the adjustable-loop group who had grade 2 pivot-shift test findings had excellent Lysholm scores.
In a meta-analysis by Connaughton et al.,[47] they compared all inside ACL reconstruction (both femoral and tibial suspensory fixation) and standard ACL reconstruction (femoral suspensory fixation and tibia aperture fixation). They found that clinical outcomes are similar between the two, even though the lowest visual analog scale scores were seen with all-inside techniques. Some studies reported a higher graft failure rate in all inside techniques, probably attributed to higher graft elongation in suspensory fixation and early return to pivoting sports before ligamentization.[48] Hence, there is no superiority among transfemoral fixation, extracortical suspensory fixation, and interference screw fixation for soft-tissue grafts in clinical outcome, even though there were significant differences in biomechanical analysis and tunnel widening.
APERTURE FIXATION
Direct or aperture fixation devices are used to compress the graft against the outer surface of the bone or wall of the bone tunnel. Aperture fixation devices include interference screws and staples.[15] Interference screws which are currently used in practice include metal (titanium) and biodegradable screws. Bioabsorbable materials include polylactic acid, poly-L-lactic acid (PLLA), or polyglycolic acid (PGA), all of which degrade and are replaced by tissue over time [Figure 3].

-
: Bioscrews with Intrasheath.
Biomechanics
Ishibashi et al., in the biomechanical analysis using porcine knees, proved that proximal graft fixation in tibia reduced anterior displacement and internal rotation of tibia as well as in situ forces of the graft itself.[49] Mayr et al., in his biomechanical study using calf tibiae, found that interference screws demonstrated higher pullout stiffness (309.5N/mm vs. 185.6 N/mm) and reduced elongation (3.33 mm vs. 6.08 mm) than adjustable loops.[43] However, the ultimate failure load was lower than adjustable loops (693N vs. 908N), which was well within the 590N range.[32,43] Scheffler et al. suggested that direct anatomic fixation should be used for soft-tissue fixation, especially on the tibial side, since it is the weakest point in all reconstructions.[35] However, this direct soft-tissue fixation had the risk of graft slippage since the mechanical engagement between the screw and the graft is less intense. Further, it has been suggested that the screws caused laceration and necrosis of soft-tissue grafts.[22] Hence, it is better to go for the application of backup or hybrid fixation, especially on the tibial fixation site. Hakimi et al. found that in UK, almost 57% of tibial fixation was most commonly done by interference screws, followed by Intrafix (31%). A bioabsorbable interference screw was used in 97% of cases for tibial side fixation.[24] Interference screws should hold the graft firmly for a minimum of 12 weeks so early rehabilitation is allowed without any concern in graft integration. Other direct methods such as clawed washer-screw combination and staples showed inferior biomechanics and increased risk of complications such as skin irritation, percutaneous ganglion with these devices.[50]
Biomechanical analysis between metal and biodegradable screws using flexor graft tendon complex in porcine knees was done by Nakano et al.[51] He found that biodegradable screws had high stiffness and low ultimate failure load than metal screws. The ultimate failure load of biodegradable screws is higher than titanium for femoral side soft-tissue graft fixation and similar for tibial side fixation. However, titanium screws produce more screw thread-induced graft laceration, especially on the femoral side than the tibial side. Hence, metal screws should not be preferred for femoral soft-tissue graft fixation. This shows the superiority of biodegradable screws over titanium screws for soft-tissue graft fixation biomechanically, especially in the tibial side.
Clinical outcome
Shen et al.,[52] in his meta-analysis, included 12 studies comparing biodegradable screws and metal screws clinically. They showed similar clinical outcomes with respect to IKDC scores, Lysholm scores, KT arthrometer testing, and infection rate. However, the biodegradable screw group had an increased rate of knee effusion (Risk ratio [RR], 2.57; P = 0.04; 421 patients in four studies).
Bone-tendon-bone (BTB) graft-interference screw fixation
Bone tendon grafts are mechanically more stable than hamstring grafts. Interference screws, either metal or biodegradable, were most commonly used for BTB graft fixation in the femoral and tibial sides. Compression between graft bone and wall of bone tunnel with the help of interference screws assists in direct bone-to-bone healing of the graft. Plominski[53] compared the clinical results of BTB graft ACL reconstruction using metal and biodegradable screws. After the follow-up of three years, they found no statistical difference in Lysholm scores and IKDC scores. However, osteolytic changes were observed in the biodegradable screws group with evidence of foreign body reaction in one patient. But Hackl et al compared polyglyconate bioscrew and titanium screw and found no evidence of increased complications for polyglyconate screw for BTB graft fixation.[54] However, for BTB graft fixation, both metal and biodegradable screws can be used, but the risk of osteolysis and foreign body reaction associated with biodegradable screws should be considered. Staerke et al. found that the most rigid graft/fixation combination was bone-patellar tendon-bone (BPTB) graft fixed with an interference screw as it demonstrated minimal graft migration compared to soft-tissue grafts fixed with EndoButton and cross-pins. Hence, the BPTB graft and interference screw combination has often been considered the “gold standard” in ACL reconstruction due to its superior mechanical stability.[22]
Not only do material properties of interference screws influence the stability of the fixation, the other parameters such as screw length, diameter, screw slope, divergence, placement of the screw, bone mineral density (BMD), and insertion torque influence the stability of the fixation.
Screw length
Stalder et al.,[55] in his study, showed that the femoral fixation strength of the interference screw significantly improved by approximately 30% if the screw is shorter than the graft end in the bone for soft-tissue grafts. However, in tibial fixation of soft-tissue graft, Weiler et al.[56] showed that increasing screw length improves fixation strength for the same diameter (23 mm–367.2N vs. 28 mm–537.4N). Hence, the length of the screw should be adequate enough to hold the graft, especially in the proximal part of the tibial tunnel, so that fixation strength is increased in the weak link of ACL reconstruction.[49,57]
Screw diameter
Graft fixation strength (367.2N vs. 479.1N) was higher when the screw diameter is more than the graft diameter by 1 mm, which is due to the press fit mechanism crushing the surrounding cancellous bone. However, there is poor engagement of thread into the bone.[15,22] Shapiro et al.[58] found that in BTB graft fixation, there was no difference in biomechanical parameters between 7 mm and 9 mm screws, and iatrogenic injuries to patellar tendon bone block were lower with 7 mm screws.
Screw slope
Daneshvarhashjin et al.[59] recently studied newly designed and fabricated two differently tapered interference screws where the diameters of both screws were equal to bone tunnel diameter in one-third of their length from screw tip, then they were gradually increased by 1 mm, in the lower slope tapered interference screw (LSTIS), and 2 mm, in the higher slope tapered interference screw (HSTIS) screws [Figure 4]. On biomechanical analysis, HSTIS showed greater graft-bone-screw construct stiffness and a lower graft laxity compared to LSTIS. Biomechanically, higher slope screws demonstrated lower contact pressure than lower slope screws in the proximal one-third region of the tibia tunnel which prevents rupture of grafts, and better contact pressure in the mid-third and outer third region, which prevents graft slippage. Hence, screw slope also determines the stability of the fixation, which needs further research in vitro and in vivo.
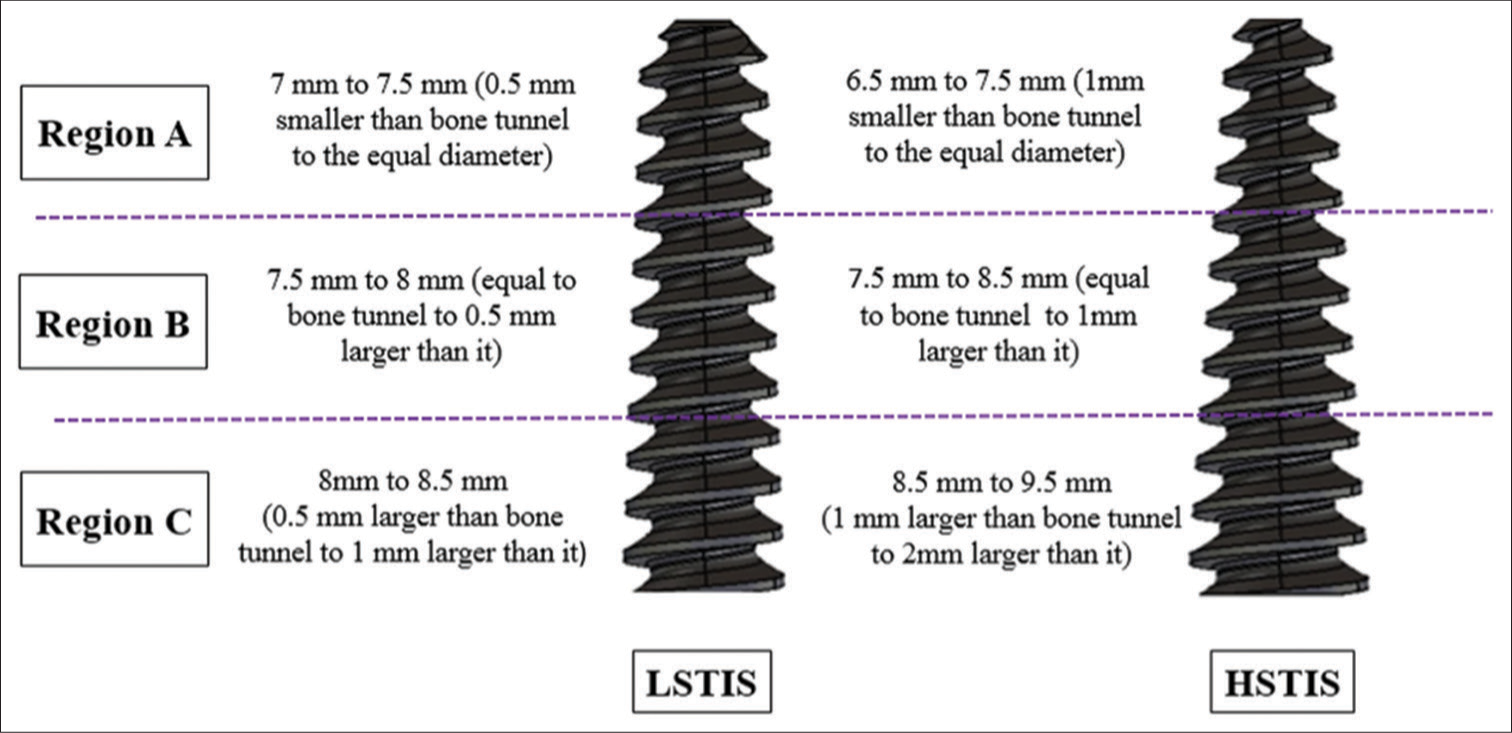
- Images of two tapered interference screws. Left side – lower slope tapered interference screw (LSTIS) and right side – higher slope tapered interference screw (HSTIS).
BMD and insertion torque
Both insertion torque and BMD were related to the maximum load the graft withstood.[60] Phillips et al.[61] found that the insertion torque was significantly higher at the distal third of the tibial tunnel (outer cortex, 8.7in/lb) than at the middle third and proximal third (joint line of the tibial tunnel – 4.3 in/lb). Lower insertion torque at the proximal third of the tibial tunnel results in lower peak load and pullout strength of the graft, and this may outweigh the proposed benefits of joint-line fixation on the tibial side, as shown by Ishibashi et al.[10] Hence, measuring BMD and insertion torque in biomechanical analysis of interference screw fixation is necessary.
Screw placement
Placement of the screw can be eccentric or concentric. The concentric placement of the Bio-Intrafix screw within its sheath ensures 360° graft-to-bone placement with better engagement. Wang et al., in their biomechanical analysis, found that the Intrafix demonstrated a higher failure load (719N vs. 476N) than biodegradable screws. Their stiffness and displacement remain the same.[15] Hence, soft-tissue fixation on the tibial side with Intrafix and biodegradable screws is able to meet physiological demands.
Divergence
Divergence of the screw from the graft may occur during insertion, especially on the femoral side. However, this deviation can be reduced by inserting the screw through the same portal, the femoral tunnel that was drilled, which can be easily done in the medial portal technique of ACL reconstruction.[61] Schroeder et al.[62] found a reduction in fixation strength if the divergence was more than 15°.
COMPLICATIONS
Transfemoral fixation
Pain due to prominent hardware is the common complication reported with cross-pins. It occurs due to the migration of cross-pins medially, laterally, or intraarticularly,[63] leading to loose body formation and chondral damage. Cross-pins provide good stability and clinical outcomes, even though they are malpositioned. However, unpredictable complications can be avoided with proper positioning of the cross-pin tunnel and the usage of correct surgical technique.
Suspensory fixation
Tunnel widening is the most common problem encountered with the usage of extracortical suspensory fixation. However, tunnel widening has not been found to have affected clinical outcomes, but it may complicate the revision of ACL reconstruction.[25] Kong et al. reported that complications include failure of the button to deploy in the proper position (i.e., it may not flush with the distal femoral cortex, or it may have deployed in the intraosseous tunnel).[64] Mae et al. found that 25.2% of patients after ACL reconstruction had tissue interposition between the EndoButton and femoral lateral cortex, and EndoButton with tissue interposition migrated more frequently than those without it after ACL reconstruction. However, clinical outcomes remained the same with the migrated EndoButton.[65] Sylvian Guy suggested direct visualization of the implant to check for proper deployment and to avoid tissue interposition which shortens the distance between ITB and button which will prevent ITB friction [Figure 5].[66]
- Soft-tissue interposition between the cortex and the button.
Interference screws
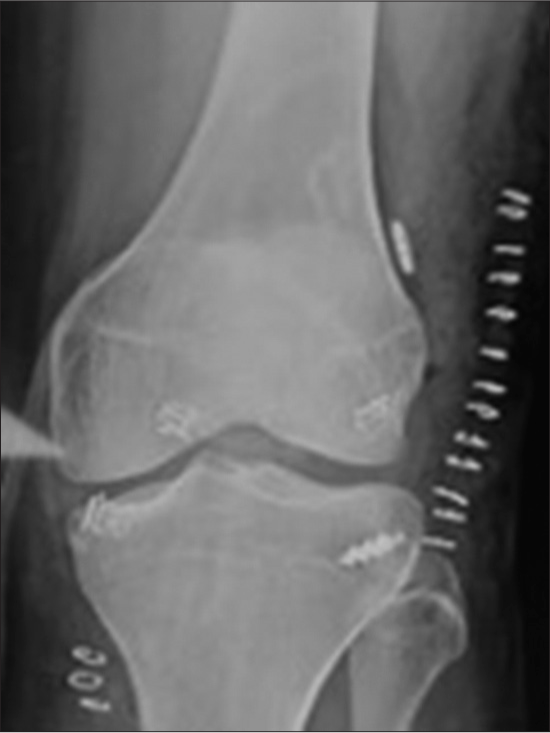
Metal interference fixation has been associated with several intraoperative complications, including graft laceration, breakage of the bone block, advancement of graft, and incorrect screw placement.[67] Even though there were cases of migration of screws in the popliteal fossa, intra-articular notch, and lateral gutter, there were no reports of migrated screws causing loss of stability[68] [Figure 6]. The probable cause for migration explained was bone resorption, incorrect placement of the tunnel or screw, incomplete insertion of the screw, and secondary twisting injury. Hence, migrated metal screws, if present and symptomatic, should be removed arthroscopically, and arthrotomy can be considered for difficult removal.
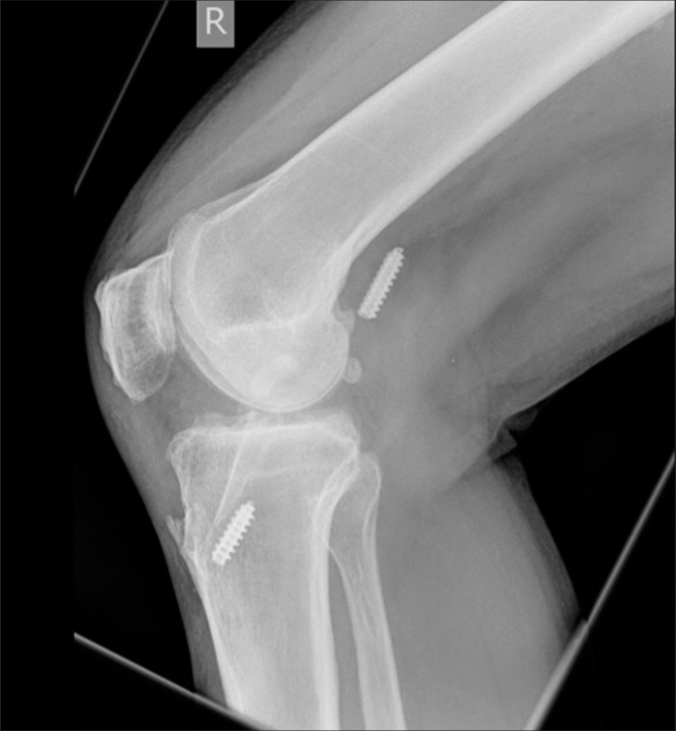
- Migration of screws in the popliteal fossa (Case courtesy of Henry Knipe, Radiopaedia.org, rID: 72407).
Bioabsorbable screws are less likely to cause graft laceration than metal screws and it won’t create ferromagnetic artifacts in MRI. It allows for easier revision surgery. However, biodegradable screws are associated with several complications due to their incomplete or prolonged degradation and incomplete ossification. Degradation is influenced by several factors which include polymer type, implant size, location, local circulation, the percentage of crystallinity, molecular weight, and surface area open to degradation.[69] PGA screws degrade rapidly and PLLA screws have longer degradation periods.[70] Macarini with MRI analysis reported that 34 of 35 poly-D-L-lactic acid (PDLLA) screws demonstrated complete degradation and ossification at the end of three years. There was a cyst-like formation at the screw site, which was considered to be a normal feature of the screw degradation process.[71]
Degradation of bioscrews occurs by hydrolysis of the hydrolytically unstable polymer. Breakdown products (glycolic acid and lactic acid) that accumulate create a locally acidic environment that stimulates resorption and inhibits bone formation.[72] In biocomposite devices, there will be associated osteoconductive components adding to bioabsorbable components, promoting bony ingrowth. When the bioabsorbable polymer portion degrades, there will be an increase in the porosity of the implant, expanding the surface area for the breakdown of calcium and phosphate.[73] This stimulates osteoblasts and increases bone production. Basic salts released by the breakdown of the osteoconductive portion counteract the acidic byproducts of the polymer, which leads to reduced resorption effects. Hence, adding an osteoconductive portion to a bioabsorbable screw has negative effects on screw degradation.[69] Gradual resorption of the screw leads to the weakening of the device, and it may fracture into fragments, producing mechanical symptoms as well as inflammatory reactions such as synovitis, effusion, cyst or granuloma formation, sterile abscess, or wound dehiscence. There are reports of fractured screw fragments migrating intraarticularly, producing mechanical symptoms and chondral damage.[74] Hence, early identification is necessary to prevent the devastating complications of chondral injury. Gonzalez-Lomas et al. reported a similar 5% incidence of pretibial cyst formation and found that none of the cysts were infectious, and the graft was well incorporated without any loss in instability.[75] As there is incomplete bone integration associated with biodegradable screws, stress risers would be created, leading to fracture. Biodegradable screws also has the demonstrated risk of fracture during insertion without any failure of reconstruction.[76]
Shen et al.[52], in their meta-analysis, revealed that more tunnel widening was observed with biodegradable screws than metal screws, especially on the femoral side, without any loss in stability. Other significant adverse events include knee effusion and screw breakage. However, functional outcome measures between the two remain the same. This does not justify the advantage of biodegradable screws over metal screws. Even though it is said to be advantageous in MRI, requesting for follow-up MRI after ACL reconstruction is not a routine clinical practice. Metal screws are said to cause graft laceration, but there was only one case report regarding the same in the meta-analysis of Shen et al.[52] Although it was claimed that biodegradable screws allow for easier revision surgery, there was no Randomised Controlled Trial (RCT) proving the same. Hence, biodegradable screws can be used for fixation as it has better biomechanical and functional outcomes, but the patient should be informed about the risks and complications associated with it.
CONCLUSION
ACL graft fixation implants available in the market currently provide acceptable clinical outcomes with minimal complications, even though there are biomechanically superior implants for femoral and tibial fixation. Every arthroscopic surgeon should have the knowledge of biomechanical analysis, clinical outcome, and complications of the implant they are using to fix the ACL graft. Implant specific complications and its management need detailed research in future and development of ideal implant should be promoted.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this review.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Current trends in the anterior cruciate ligament part II: Evaluation, surgical technique, prevention, and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2022;30:34-51.
- [CrossRef] [Google Scholar]
- Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47:221-32.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative pull-out and cyclic-loading strength tests of anchorage of hamstring tendon grafts in anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:621-5.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior cruciate ligament autograft maturation on sequential postoperative MRI is not correlated with clinical outcome and anterior knee stability. Knee Surg Sports Traumatol Arthrosc. 2022;30:3258-67.
- [CrossRef] [PubMed] [Google Scholar]
- The phenomenon of “ligamentization”: Anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4:162-72.
- [CrossRef] [PubMed] [Google Scholar]
- The “ligamentization” process in human anterior cruciate ligament reconstruction with autogenous patellar and hamstring tendons: A biochemical study. Am J Sports Med. 2005;33:1166-73.
- [CrossRef] [PubMed] [Google Scholar]
- Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1999;7:9-14.
- [CrossRef] [PubMed] [Google Scholar]
- Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197-205.
- [CrossRef] [PubMed] [Google Scholar]
- The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14:449-62.
- [CrossRef] [PubMed] [Google Scholar]
- Graft incorporation within the tibial bone tunnel after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft. Am J Sports Med. 2001;29:473-9.
- [CrossRef] [PubMed] [Google Scholar]
- Tendon healing in bone tunnel after human anterior cruciate ligament reconstruction: A systematic review of histological results. J Knee Surg. 2019;32:454-62.
- [CrossRef] [PubMed] [Google Scholar]
- Graft healing after anterior cruciate ligament reconstruction (ACLR) Asia Pac J Sports Med Arthrosc Rehabil Technol. 2021;25:8-15.
- [CrossRef] [PubMed] [Google Scholar]
- Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795-803.
- [CrossRef] [PubMed] [Google Scholar]
- Hamstring graft motion in the femoral bone tunnel when using titanium button/polyester tape fixation. Knee Surg Sports Traumatol Arthrosc. 1999;7:215-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fixation of the graft in reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2005;87:593-603.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and biomechanics of the native and reconstructed anterior cruciate ligament: Surgical implications. J Bone Joint Surg Am. 2017;99:438-45.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern of anterior cruciate ligament force in normal walking. J Biomech. 2004;37:797-805.
- [CrossRef] [PubMed] [Google Scholar]
- Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72:557-67.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of cruciate-ligament loading during rehabilitation exercise. Clin Biomech (Bristol, Avon). 1998;13:403-13.
- [CrossRef] [Google Scholar]
- Optimal graft stiffness and pre-strain restore normal joint motion and cartilage responses in ACL reconstructed knee. J Biomech. 2016;49:2566-76.
- [CrossRef] [PubMed] [Google Scholar]
- On measuring implant fixation stability in ACL reconstruction. Sensors (Basel). 2021;21:6632.
- [CrossRef] [PubMed] [Google Scholar]
- ACL graft migration under cyclic loading. Knee Surg Sports Traumatol Arthrosc. 2010;18:1065-70.
- [CrossRef] [PubMed] [Google Scholar]
- All-inside anterior cruciate ligament graft-link technique: Second-generation, no-incision anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:717-27.
- [CrossRef] [PubMed] [Google Scholar]
- J Bone Joint Surg Br. 94B. Current UK Practice; 2012. ACL Reconstruction-Current UK Practice. :42.
- [Google Scholar]
- Clinical and functional outcomes after anterior cruciate ligament reconstruction using cortical button fixation versus transfemoral suspensory fixation: A systematic review of randomized controlled trials. Arthroscopy. 2014;30:1491-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bone tunnel enlargement after anterior cruciate ligament reconstruction: Fact or fiction? Knee Surg Sports Traumatol Arthrosc. 1998;6:231-40.
- [CrossRef] [PubMed] [Google Scholar]
- Tunnel widening after hamstring anterior cruciate ligament reconstruction is influenced by the type of graft fixation used: A prospective randomized study. Arthroscopy. 2005;21:1337-41.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of early motion on tibial tunnel widening after anterior cruciate ligament replacement using hamstring tendon grafts. Arthroscopy. 2004;20:572-80.
- [CrossRef] [PubMed] [Google Scholar]
- Femoral tunnel enlargement after anatomic ACL reconstruction: A biological problem? Knee Surg Sports Traumatol Arthrosc. 2010;18:1189-94.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective evaluation of tunnel enlargement in anterior cruciate ligament reconstruction with hamstrings: Extracortical versus anatomical fixation. Knee Surg Sports Traumatol Arthrosc. 2002;10:80-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between different femoral fixation devices for ACL reconstruction with doubled hamstring tendon graft: A biomechanical analysis. Arthroscopy. 2006;22:660-8.
- [CrossRef] [PubMed] [Google Scholar]
- The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: Femoral site. Am J Sports Med. 2003;31:174-81.
- [CrossRef] [PubMed] [Google Scholar]
- Biomechanical comparison of Cross-pin and Endobutton-CL femoral fixation of a flexor tendon graft for anterior cruciate ligament reconstruction--a porcine femur-graft-tibia complex study. J Surg Res. 2010;161:282-7.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of clinical outcomes of two methods of femoral hamstring graft pin fixation in anterior cruciate ligament reconstruction. In: Proceedings of Singapore healthcare. 2013. p. :248-52.
- [CrossRef] [Google Scholar]
- Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: The impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18:304-15.
- [CrossRef] [PubMed] [Google Scholar]
- Stress fractures of the femur after ACL reconstruction with transfemoral fixation. Knee Surg Sports Traumatol Arthrosc. 2006;14:1148-50.
- [CrossRef] [PubMed] [Google Scholar]
- Cross pins versus endobutton femoral fixation in hamstring anterior cruciate ligament reconstruction: Minimum 4-year follow-up. Knee Surg Relat Res. 2012;24:34-9.
- [CrossRef] [PubMed] [Google Scholar]
- Femoral interference screw fixation of hamstring and quadriceps tendons for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25:1241-8.
- [CrossRef] [PubMed] [Google Scholar]
- Suspensory fixation of grafts in anterior cruciate ligament reconstruction: A biomechanical comparison of 3 implants. Arthroscopy. 2009;25:767-76.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical properties of suspensory fixation devices for anterior cruciate ligament reconstruction: Comparison of the fixed-length loop device versus the adjustable-length loop device. Knee. 2014;21:743-8.
- [CrossRef] [PubMed] [Google Scholar]
- Femoral suspension devices for anterior cruciate ligament reconstruction: Do adjustable loops lengthen? Am J Sports Med. 2014;42:343-9.
- [CrossRef] [PubMed] [Google Scholar]
- The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: Tibial site. Am J Sports Med. 2003;31:182-8.
- [CrossRef] [PubMed] [Google Scholar]
- Biomechanical comparison of 2 anterior cruciate ligament graft preparation techniques for tibial fixation: Adjustable-length loop cortical button or interference screw. Am J Sports Med. 2015;43:1380-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hamstring anterior cruciate ligament reconstruction: A comparison of bioabsorbable interference screw and endobutton-post fixation. Arthroscopy. 2004;20:122-8.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective comparison of 3 hamstring ACL fixation devices--Rigidfix, BioScrew, and Intrafix--randomized into 4 groups with 2 years of follow-up. Am J Sports Med. 2009;37:699-706.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and radiological outcomes after hamstring anterior cruciate ligament reconstructions: Comparison between fixed-loop and adjustable-loop cortical suspension devices. Am J Sports Med. 2017;45:826-31.
- [CrossRef] [PubMed] [Google Scholar]
- All-inside ACL reconstruction: How does it compare to standard ACL reconstruction techniques? J Orthop. 2017;14:241-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and functional outcome of all-inside anterior cruciate ligament reconstruction at a minimum of 2 years' follow-up. Arthroscopy. 2016;32:332-7.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of anterior cruciate ligament graft fixation site at the tibia on knee stability: Evaluation using a robotic testing system. Arthroscopy. 1997;13:177-82.
- [CrossRef] [PubMed] [Google Scholar]
- Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27:35-43.
- [CrossRef] [PubMed] [Google Scholar]
- Interference screw fixation of doubled flexor tendon graft in anterior cruciate ligament reconstruction-biomechanical evaluation with cyclic elongation. Clin Biomech (Bristol, Avon). 2000;15:188-95.
- [CrossRef] [Google Scholar]
- Bioabsorbable versus metallic interference screw fixation in anterior cruciate ligament reconstruction: A meta-analysis of randomized controlled trials. Arthroscopy. 2010;26:705-13.
- [CrossRef] [PubMed] [Google Scholar]
- Fixation of patellar tendon bone graft in reconstruction of patellar ligaments. Comparison of bioabsorbable and metal interference screws--results of treatment. Ortop Traumatol Rehabil. 2008;10:44-53.
- [Google Scholar]
- Transplantatfixation bei der vorderen Kreuzbandrekonstruktion. Metall-vs. bioresorbierbare Polyglykonatinterferenzschraube--Eine prospektive randomisierte Studie von 40 Patienten [Transplant fixation by anterior cruciate ligament reconstruction. Metal vs. bioabsorbable polyglyconate interference screw. A prospective randomized study of 40 patients] Unfallchirurg. 2000;103:468-74.
- [CrossRef] [PubMed] [Google Scholar]
- Interference screws should be shorter than the hamstring tendon graft in the bone tunnel for best fixation. Knee Surg Sports Traumatol Arthrosc. 2013;21:584-8.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28:356-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclic pull-out strength of hamstring tendon graft fixation with soft tissue interference screws. Influence of screw length. Am J Sports Med. 1999;27:778-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of pullout strength for seven-and nine-millimeter diameter interference screw size as used in anterior cruciate ligament reconstruction. Arthroscopy. 1995;11:596-9.
- [CrossRef] [PubMed] [Google Scholar]
- Can the body slope of interference screw affect initial stability of reconstructed anterior cruciate ligament?: An in-vitro investigation. BMC Musculoskelet Disord. 2021;22:556.
- [CrossRef] [PubMed] [Google Scholar]
- Insertion torque pullout strength relationship of soft tissue tendon graft tibia tunnel fixation with a bioabsorbable interference screw. Arthroscopy. 2004;20:379-84.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of interference screw insertion torque with depth of placement in the tibial tunnel using a quadrupled semitendinosus-gracilis graft in anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:1026-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reduction of femoral interference screw divergence during endoscopic anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:41-8.
- [CrossRef] [PubMed] [Google Scholar]
- Complications following hamstring anterior cruciate ligament reconstruction with femoral cross-pin fixation. Arthroscopy. 2005;21:762.
- [CrossRef] [PubMed] [Google Scholar]
- An unidentified pitfall of Endobutton use: Case report. Knee Surg Sports Traumatol Arthrosc. 2002;10:247-9.
- [CrossRef] [PubMed] [Google Scholar]
- Migration of EndoButton after anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1528-35.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic confirmation of femoral button deployment prevents soft tissue interposition in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2022;30:2251-8.
- [CrossRef] [PubMed] [Google Scholar]
- Interference screw divergence in endoscopic anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:45-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intraarticular migration of a femoral interference fit screw. A complication of anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:268-71.
- [CrossRef] [PubMed] [Google Scholar]
- Bioabsorbable versus titanium screws in anterior cruciate ligament reconstruction using hamstring autograft: A prospective, blinded, randomized controlled trial with 5-year follow-up. Am J Sports Med. 2015;43:1893-901.
- [CrossRef] [PubMed] [Google Scholar]
- Bio-interference screw cyst formation in anterior cruciate ligament reconstruction--10-year follow up. Knee. 2012;19:644-7.
- [CrossRef] [PubMed] [Google Scholar]
- MRI in ACL reconstructive surgery with PDLLA bioabsorbable interference screws: Evaluation of degradation and osteointegration processes of bioabsorbable screws. Radiol Med. 2004;107:47-57.
- [Google Scholar]
- Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335-46.
- [CrossRef] [PubMed] [Google Scholar]
- Chemical synthesis of poly(lactic-coglycolic acid)/hydroxyapatite composites for orthopaedic applications. Acta Biomater. 2006;2:277-86.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous locking of the knee after anterior cruciate ligament reconstruction as a result of a broken tibial fixation device. Arthroscopy. 2008;24:1195-7.
- [CrossRef] [PubMed] [Google Scholar]
- Is the etiology of pretibial cyst formation after absorbable interference screw use related to a foreign body reaction? Clin Orthop Relat Res. 2011;469:1082-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bioabsorbable interference screw failure in anterior cruciate ligament reconstruction: A case series and review of the literature. Knee. 2015;22:256-61.
- [CrossRef] [PubMed] [Google Scholar]







