Translate this page into:
Management and reconstruction strategies for multiligament knee injuries: Advances in diagnosis, surgical techniques, and rehabilitation
*Corresponding author: Robert F. LaPrade, Twin Cities Orthopedics, Edina, Minnesota, United States. laprademdphd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sleem B, Nassar JE, Tollefson LV, LaPrade RF. Management and reconstruction strategies for multiligament knee injuries: Advances in diagnosis, surgical techniques, and rehabilitation. J Arthrosc Surg Sports Med. doi: 10.25259/JASSM_62_2024
Abstract
Background and Aims
Multiligament knee injuries (MLKIs) are complex, high-stakes injuries often resulting from high-energy trauma, requiring precise diagnosis, tailored surgical planning, and structured rehabilitation to restore stability and function. This review examines strategies for MLKI management, highlighting Schenck’s classification system, physical assessment, imaging techniques, and graft choices.
Materials and Methods
A comprehensive literature search was conducted using PubMed, Embase, and Cochrane Library to identify studies on MLKIs, focusing on anatomy, diagnostic methods, and treatment approaches.
Results
Operative timing - acute, staged, and delayed - and decision-making between ligament repair and reconstruction are evaluated to optimize patient outcomes. In addition, advancements in graft selection and surgical techniques, such as double-bundle posterior cruciate ligament reconstruction, further enhance knee kinematics and reduce graft failure risk. The rehabilitation protocol focuses on balancing protection, early mobilization, and progressive strengthening to facilitate recovery and minimize complications like arthrofibrosis.
Conclusion
By integrating comprehensive treatment planning and patient-specific rehabilitation, MLKI management can significantly improve functional outcomes and patient satisfaction.
Keywords
Graft selection
Knee reconstruction
Multiligament knee injury
Surgical techniques
Tensioning sequence
INTRODUCTION
Multiligament knee injuries (MLKIs) are severe injuries involving the disruption of two or more major knee ligament complexes, often caused by high-energy trauma such as sport collisions or road accidents.[1] These injuries are challenging to manage due to the knee’s intricate stabilizing structures, including the anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), the posteromedial corner (PMC), and posterolateral corner (PLC). The PMC consists of the medial collateral ligament (MCL) and posterior oblique ligament (POL), while the PLC includes the fibular collateral ligament (FCL), popliteus tendon (PLT), and popliteofibular ligament (PFL).[2,3]
An effective management requires an individualized approach that consists of surgical treatment and a proper rehabilitation. Surgical intervention often improves functional outcomes and stability but one must also consider patient-specific factors such as age, activity level, and associated injuries that could impact the magnitude of improvement.[4] Moreover, rehabilitation is equally important with early mobilization while ensuring that the surgical site remains protected to ensure an optimal recovery.[5]
This narrative review provides a comprehensive overview of MLKI treatment strategies, emphasizing reconstruction techniques. It examines surgical versus non-surgical approaches, optimal timing, and reconstruction-specific considerations, offering evidence-based guidance for personalized treatment plans and improved outcomes.
METHODS
A comprehensive literature search was conducted using PubMed, Embase, and Cochrane Library to identify studies on MLKIs, focusing on anatomy, diagnostic methods, and treatment approaches. The search terms included “multiple ligament knee injury,” “physical examination,” “imaging studies,” “grafts,” “reconstruction,” “rehabilitation,” and “treatment outcomes.” Studies were included if they addressed MLKI anatomy, diagnostic techniques, physical examination and imaging methods, treatment strategies, and postoperative rehabilitation protocols.
Studies were included if they met the following criteria: (1) Focused on MLKI anatomy, diagnostic techniques, or treatment strategies; (2) provided clinical, imaging, or surgical data on physical examination, imaging methods, or reconstruction; (3) reported on postoperative rehabilitation protocols or treatment outcomes; and (4) were peer-reviewed articles published in English. Moreover, studies were excluded if they were commentaries or articles that did not directly discuss MLKIs.
ANATOMY OF THE FOUR PRINCIPAL KNEE LIGAMENT STRUCTURES
The ACL, PCL, PMC, and PLC are key stabilizers of the knee, each with a specific function.[6] The ACL prevents anterior tibial translation and controls anterolateral rotation. The PCL limits posterior tibial displacement and enhances rotational stability. The PMC provides medial reinforcement, countering valgus stress and anteromedial rotation. The PLC resists varus forces, external rotation, and posterior translation.
The Schenck’s classification system [Table 1] is widely used to systematically categorize the severity and complexity of MLKIs. It classifies injuries based on specific ligament combinations and associated structural damage, aiding in diagnosis, treatment planning, and prognostic assessment.[7,8] Among these classifications, KD-IIIL injuries are particularly noteworthy due to their frequent association with vascular injuries including damage to the popliteal artery which would require an immediate vascular evaluation to prevent severe complications such as limb ischemia or even amputation.[6]
| Classification | Description | Injured structures |
|---|---|---|
| KD-I | Isolated ligament injury | ACL or PCL |
| KD-II | Bicruciate ligament injury | ACL and PCL |
| KD-III-M | Bicruciate injury with medial-sided disruption | ACL, PCL, and MCL |
| KD-III-L | Bicruciate injury with lateral-sided disruption | ACL, PCL, and FCL |
| KD-IV | Combined bicruciate and collateral ligament injuries | ACL, PCL, MCL, and FCL |
| KD-V | Dislocation with periarticular fracture | ACL, PCL, MCL, FCL, and periarticular bones |
ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, MCL: Medial collateral ligament, FCL: Fibular collateral ligament, M: Medial, L: Lateral
ASSESSMENT OF MLKIS
Assessment of neurovascular status
Guidelines for managing knee dislocations in the acute setting emphasize a thorough vascular assessment.[4,9,10] This begins with a physical examination, including palpation of the dorsalis pedis and posterior tibial pulses, and measurement of the ankle-brachial index (ABI).[4] An ABI <0.9 necessitates further investigation using arterial duplex ultrasonography or computed tomography angiography, with vascular surgical consultation required if arterial injury is confirmed.[11] For patients with absent or diminished pulses, immediate joint reduction is followed by reassessment. If pulses remain compromised post-reduction, vascular surgical exploration is necessary.[11] If pulses return, management is guided by ABI measurements and may include observation with serial neurovascular checks or angiography.[11,12] Conversely, a normal physical examination and ABI >0.9 provide 100% sensitivity and negative predictive value for ruling out vascular injury.[12] Serial neurovascular monitoring for up to 48 h is essential, with a low threshold for pressure measurements and fasciotomy to address compartment syndrome.[9] Timely intervention within 6-8 h is critical to prevent irreversible ischemia and optimize outcomes.[13]
Physical examination
After confirming neurovascular status, the physical examination begins with careful inspection of the knee for signs of trauma such as lacerations, swelling, or bruising, which provide clues about the mechanism and extent of injury.[14] Swelling often indicates hemarthrosis, while bruising patterns help localize trauma. Lacerations must be evaluated for joint penetration or infection risk. Range of motion (ROM) is then assessed for limitations or abnormalities.[14] A locked knee suggests a bucket handle meniscus tear, hyperextension indicates combined PLC and ACL injuries, and restricted flexion may signify swelling, intra-articular pathology, or capsular involvement.[13,15,16] In the acute setting, swelling and muscle spasms may limit the ROM assessment. Pain control and cautious handling are essential to avoid exacerbating the injury. This initial assessment guides further testing while minimizing the risk of exacerbating damage. A thorough MLKI examination includes grading ligament injuries using the Hughston Classification: Grade 1 (mild injury with minimal laxity), Grade 2 (moderate injury with noticeable laxity), and Grade 3 (severe injury with complete disruption and significant instability).[17,18] However, subjective gapping often overestimates the true amount seen on stress radiographs.
All physical examination tests should be performed in clinic but should be validated in the examination under anesthesia. Similar to stress radiographs, patient guarding or muscle spasms in acute phase may reduce reliability compared to chronic cases or the examination under anesthesia. The Lachman test evaluates ACL stability by assessing anterior tibial translation. A positive test, indicated by increased translation without a defined endpoint, suggests an ACL tear. Moreover, care is needed to avoid a pseudo-Lachman which can occur with PCL injuries. The pivot shift test identifies dynamic instability, where the knee subluxes anterolaterally in extension and reduces during flexion [Figure 1].[19,20] Varus and valgus stress tests assess lateral and medial stability at 20-30° and full extension, respectively, with positive tests typically indicating an FCL or MCL tear [Figure 2].[19] The “dimple sign,” characterized by medial skin invagination or button-holing, is a specific indicator of medial-sided injury, such as an MCL tear.[21] This sign occurs due to entrapment of soft tissue and is often seen in acute injuries. The posterior drawer test evaluates PCL integrity at 90° flexion. Increased posterior translation without a defined endpoint suggests a PCL tear, with a tibial neutral position critical for accuracy [Figure 3],[19,22] while the quadriceps active test shows tibial reduction during quadriceps contraction.[20,23] The dial test measures external tibial rotation at 30° and 90° to identify PLC or PMC injuries. A difference of ≥10° at 30° suggests PLC or PMC involvement, while positivity at both angles indicates combined PLC and PCL injury or grade 3 PMC injury. Posterolateral tibial subluxation suggests PLC injury, while anteromedial subluxation indicates a PMC injury [Figure 4].[24] In evaluating MLKIs, advanced tests like the pivot shift test and dial test play a critical role in assessing rotational and ligamentous stability. Their relevance lies in their ability to detect subtle instabilities that may not be apparent with standard anterior-posterior stability tests.
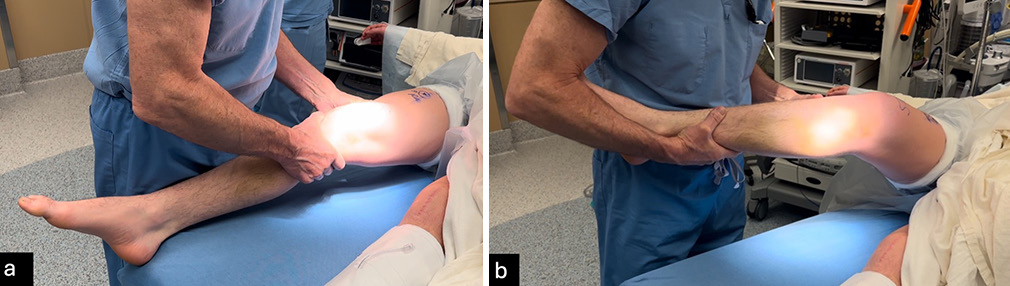
- (a) The Lachman test assesses the stability of the anterior cruciate ligament (ACL) by evaluating anterior tibial movement relative to the femur. The examiner stabilizes the femur laterally with one hand while applying an anterior force to the tibia medially. Excessive tibial translation without a firm endpoint strongly indicates an ACL rupture and potential ligamentous instability. (b) The pivot shift test examines dynamic knee instability commonly associated with ACL tears. The test begins with the knee in flexion, where one hand stabilizes the distal thigh, and the other controls the tibia. As the knee is gradually extended, the tibia is externally rotated, and a valgus force is applied. A positive result is characterized by a noticeable or palpable clunk, indicating rotational instability due to ligament insufficiency.
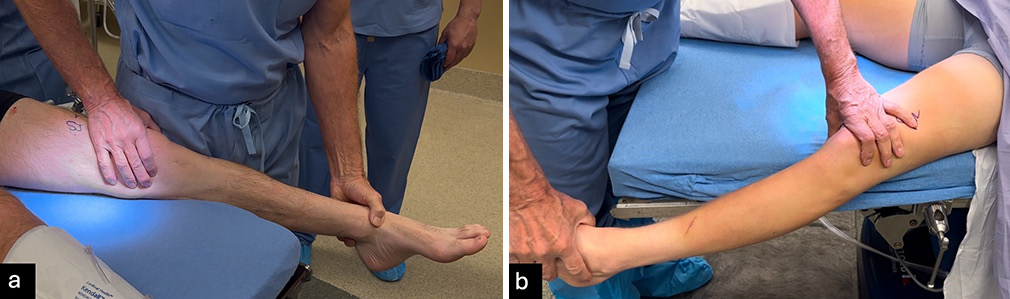
- (a) The valgus stress test evaluates the integrity of the medial collateral ligament and supporting posteromedial structures, while (b) the varus stress test assesses the fibular collateral ligament and posterolateral corner. Both assessments are performed with the patient lying supine, and the distal thigh stabilized. For the valgus test, the medial joint line is palpated as a valgus force is applied to the foot or ankle. Similarly, the varus test involves palpating the lateral joint line while applying a varus force. Each test is conducted with the knee positioned in 20-30° flexion and full extension to evaluate for potential ligamentous or cruciate injuries. A positive result is indicated by increased joint laxity or the absence of a firm endpoint when compared to the uninjured contralateral knee.
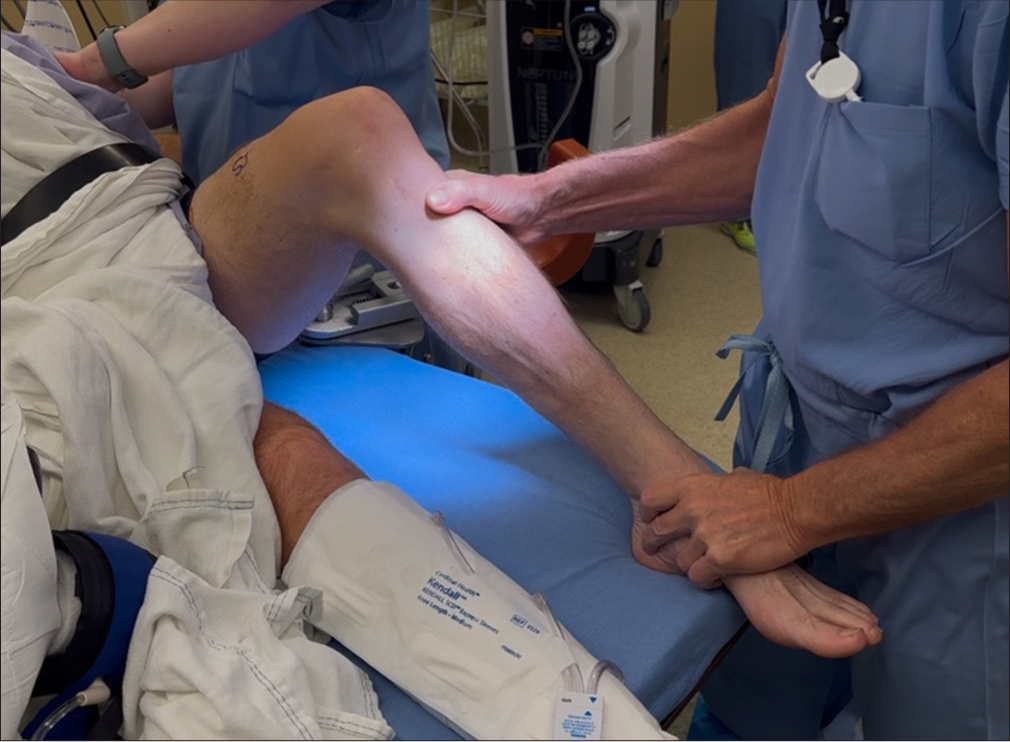
- The posterior sag test is conducted with the patient lying supine and the knee flexed to 90°. The examiner evaluates the alignment of the medial tibial plateau (MTP) in relation to the medial femoral condyle (MFC), either by visual inspection from the side or by palpating the joint line. Under normal conditions, the MTP sits approximately 1 cm anterior to the MFC; any noticeable posterior displacement indicates a posterior cruciate ligament (PCL) deficiency. In the quadriceps active test, the examiner stabilizes the patient’s ankle while monitoring tibial alignment. The patient is asked to contract the quadriceps muscles, and an anterior tibial shift exceeding 2 mm during contraction confirms the presence of PCL insufficiency.

- The dial test is used to evaluate external tibial rotation at both 30° and 90° of knee flexion, aiding in the diagnosis of posterolateral corner injuries and potential posterior cruciate ligament involvement. The test is performed with the patient lying supine. The examiner stabilizes the distal thigh to isolate knee movement while applying an external rotational force to the tibia. Comparing the affected knee to the contralateral, uninjured side helps determine the degree of instability and pinpoint the structures involved. Accurate assessment requires careful control of rotational force to avoid false positives or underestimating the injury.
Imaging
Plain radiography
Imaging evaluation for MLKIs begins with plain radiographs to assess bony structures, alignment, and injuries. The anteroposterior view identifies fractures, dislocations, and joint space narrowing, while the lateral view detects tibial subluxation, tibial eminence avulsion fractures, and tibial slope.[25] The standing flexion (Rosenberg) view at 45° flexion visualizes the posterior joint space, identifying subtle osteochondral injuries or degenerative changes.[26] Long-leg alignment views assess the mechanical axis, detecting varus or valgus deformities affecting treatment.[27] The patellar sunrise view evaluates patellar tracking, cartilage wear, and fractures or dislocations.[28]
Stress radiography
Stress radiographs offer quantitative assessments of ligament deficiencies and are highly reliable, particularly for chronic MLKIs.[29] For example, valgus stress radiographs are used to evaluate medial injuries, with side-to-side differences (SSD) of 3.2 mm at 20° flexion indicating grade 3 MCL tears, and differences exceeding 9.8 mm suggesting complete PMC injuries [Figure 5].[4] Varus stress radiographs identify isolated FCL tears (2.2-2.7 mm of gapping) or severe PLC injuries (>4.0 mm of gapping).[4,30] In chronic cases, varus stress radiography outperforms magnetic resonance imaging (MRI) for FCL injuries, as the FCL may heal in a lengthened, nonfunctional state despite appearing intact on MRI [Figure 6].[30] Stress radiographs are particularly valuable for chronic MLKIs, objectively measuring ligament stability.[31] ACL stress radiographs quantify anterior tibial translation, aiding in diagnosing ACL insufficiency and monitoring stability pre and postoperatively [Figure 7].[32] Kneeling posterior stress radiography assesses PCL injuries, with SSD values indicating partial (<8 mm), complete (8-11 mm), or combined (>12 mm) injuries [Figure 8].[29] These techniques are quick, cost-effective, and useful, though acute pain may limit their application.[4] In the acute setting, stress radiographs may be more challenging due to patient guarding and pain but should still be attempted. If the patient cannot properly perform the stress radiographs, they should not be used in diagnosis decision-making.

- Valgus stress radiographs taken at 20° of knee flexion are utilized to assess medial knee injuries by measuring side-to-side differences (SSD) in joint gapping. An SSD of 3.2 mm is indicative of a grade III tear of the superficial medial collateral ligament (MCL), while an SSD of 9.8 mm suggests a complete injury to the posteromedial corner (PMC). In the presented case, joint gapping of the (b) left knee minus joint gapping of the (a) right knee results in a SSD of 6.6 mm which points to a high-grade MCL injury of the left knee, likely involving significant ligamentous damage but without complete PMC disruption. This intermediate SSD highlights the need for a detailed evaluation to determine the extent of associated structural injuries.
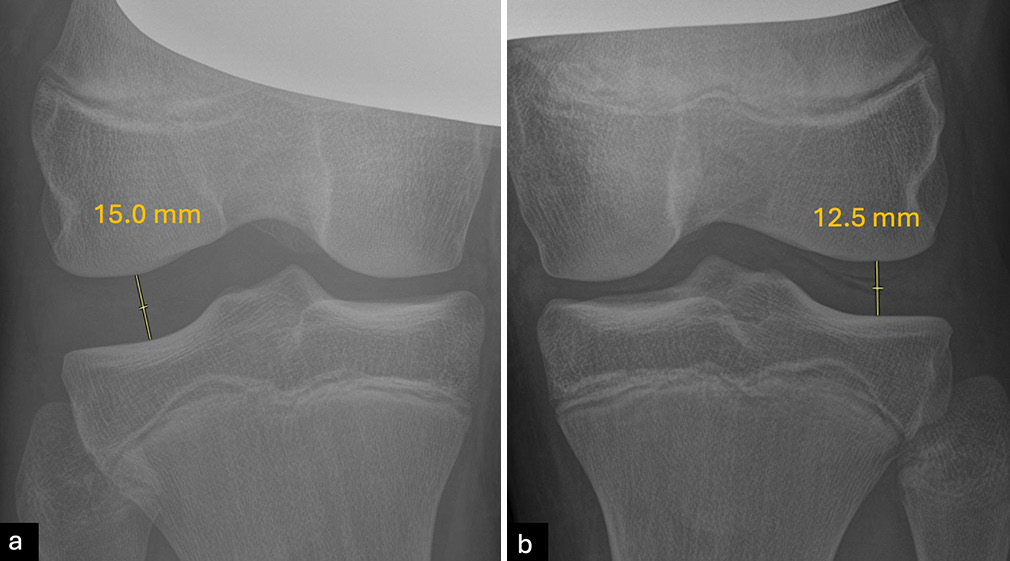
- Varus stress radiographs at 20° of knee flexion are an effective diagnostic tool for assessing valgus gapping related to fibular collateral ligament (FCL) or posterolateral corner (PLC) injuries. A side-to-side difference (SSD) in varus gapping between 2.2 mm and 4 mm typically indicates an isolated grade III (complete) tear of the FCL, while an SSD greater than 4 mm is consistent with a complete PLC injury. In the current case, joint gapping of (a) 15 mm on the right knee minus (b) 12.5 mm on the left knee results in a SSD of 2.5 mm suggesting an isolated high-grade FCL of the right knee tear without significant involvement of the PLC.
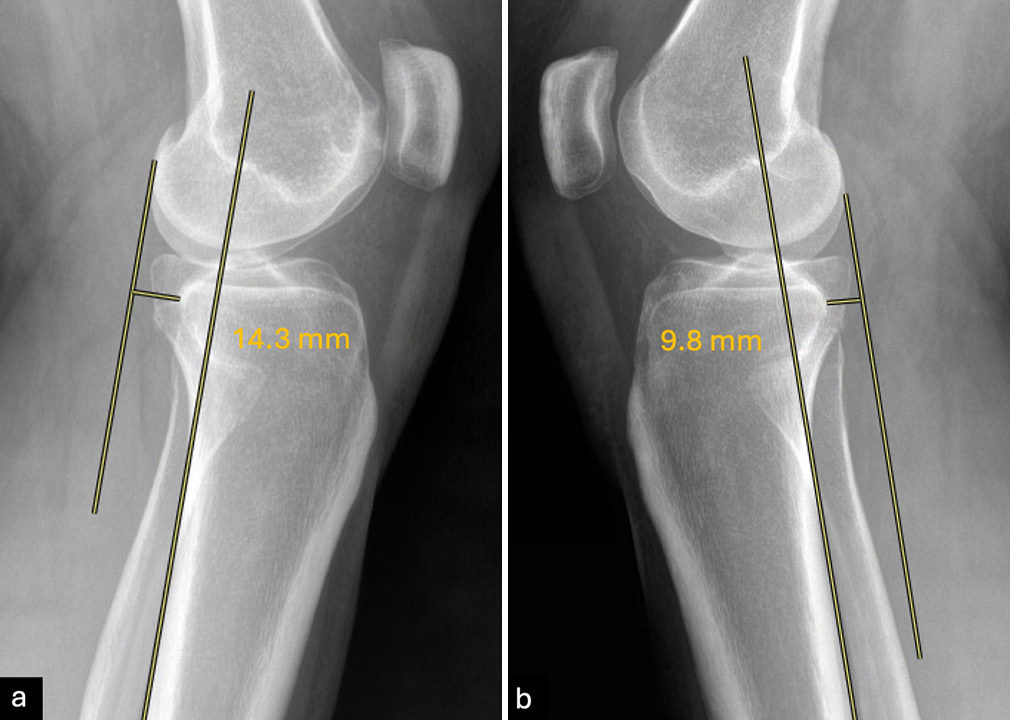
- Bilateral stress radiographs at 20° of knee flexion are used to evaluate anterior cruciate ligament (ACL) integrity. Parallel lines are drawn along the posterior edge of the tibial diaphysis and the lateral femoral condyle. A side-to-side difference (SSD) in anterior tibial translation (ATT) >3 mm indicates an ACL rupture. In this case, ATT of (a) 14.3 mm on the left knee minus ATT of (b) 9.8 on the right knee results in an SSD of 4.5 mm confirming the presence of an ACL tear of the left knee.
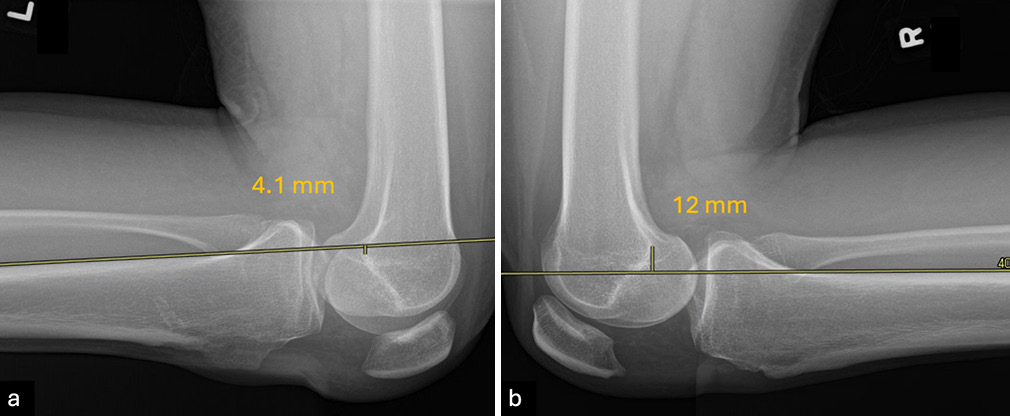
- Lateral kneeling posterior stress radiographs are used to evaluate posterior cruciate ligament (PCL) laxity by measuring posterior tibial translation (PTT) in both knees. The patient kneels with the proximal tibial joint line positioned at the edge of a table or support apparatus. A parallel line is drawn along the posterior tibial cortex, starting at least 15 cm distal to the joint line, and a perpendicular line is extended to the posterior point of the Blumensaat line. The distance measured represents the PTT for each knee, and the side-to-side difference (SSD) quantifies the extent of translation. An SSD of 0-8 mm suggests a partial PCL tear, 8-11 mm indicates a complete tear, and >12 mm suggests combined injuries. In this case, the (a) left knee has a PTT of 4.1 mm while the (b) right knee has a PTT of 12 mm, adding these together results in a SSD of 16.1 mm consistent with a full PCL tear with potential combined injuries.
MRI
In acute cases (within 3 weeks), hemarthrosis and pain can hinder diagnosis, making MRI indispensable. MRI offers nearly 100% sensitivity for MLKIs and identifies associated meniscal and chondral injuries.[33] Geeslin and LaPrade[34] reported MRI-lucent bone bruises in ~80% of PLC injuries, often on the anteromedial femoral condyle, strongly suggesting PLC involvement until proven otherwise. MRI also aids preoperative planning, as emphasized by Porrino et al.,[35] contributing to better postoperative outcomes. Goiney et al.[36] highlighted MRI’s role in guiding intraoperative decisions, particularly in selecting optimal surgical approaches for PCL injuries.
Associated injuries
Nerve and vascular injuries are significant concerns in MLKIs alongside ligament damage. Nerve injuries, particularly to the common peroneal nerve, occur in up to 25% of cases.[6] Becker et al.[37] found that MRI could detect 70% of peroneal nerve injuries, revealing issues ranging from surrounding edema to complete nerve tears. Vascular injuries, occurring in 3.3-18% of high-velocity MLKIs, are often linked to PLC injuries, posterior dislocations, and concurrent peroneal nerve damage.[38] The popliteal artery is the most commonly affected vessel, with Medina et al.[6] reporting it involved 76% of cases, where 80% required a vascular repair.
Beyond neurovascular compromise, MLKIs are often linked to cartilage damage, meniscal tears, and both intra- and periarticular fractures.[4] Multiple studies have documented meniscal injury rates in MLKI cases, with reports ranging from 15% to 55%.[10,39]
Complications and management
Complications following MLKIs can significantly affect outcomes, and prompt recognition and management are crucial. As mentioned, neurovascular injuries, particularly involving the popliteal artery or common peroneal nerve, are critical concerns. Immediate vascular assessment using ankle-brachial pressure index and imaging is essential, with surgical intervention required in cases of ischemia.[40] Nerve injuries may initially be managed conservatively, with surgical options like tendon transfer considered if deficits persist.[40]
Arthrofibrosis, or joint stiffness from scar tissue, can be mitigated by early, controlled range-of-motion exercises.[41] Severe cases may require surgical lysis of adhesions. Graft failure, often due to technical issues or premature activity, highlights the importance of anatomical reconstruction and individualized rehabilitation.[42] Revision surgeries may be necessary if failure occurs. Postoperative infections are rare but serious and can potentially be avoided by requiring adherence to sterile techniques during surgery. If infections do arise, they should be treated early with antibiotics or surgical debridement. Finally, chronic instability, even after surgery, underscores the need for meticulous surgical planning, precise technique, and diligent rehabilitation. Persistent instability may necessitate additional procedures.
TREATMENT OF MLKIS
Historically, treating patients with a MLKI involved inconsistent immobilization periods at various degrees of knee flexion. This approach yielded varied outcomes, as immobilization duration inversely impacted post-treatment mobility.[42] Extended immobilization often led to a more stable knee, but with limited active and passive motion, while shorter immobilization periods allowed for near-normal movement but frequently compromised stability.[43] However, data over the past few decades have shown that operative treatment of MLKI is associated with significantly improved functional outcomes when compared to non-operative treatment.[40] Specifically, meta-analyses have demonstrated significantly higher Lysholm scores, Tegner scores, and International Knee Documentation Committee (IKDC) scores, with conflicting results when it comes to rates of return to work and pre-injury sports activities, as well as knee ROM.[44]
TIMING OF SURGERY
While surgical treatment is generally favored for MLKIs, the timing remains a topic of debate, with three main approaches: Acute, staged, and delayed.[44,45] Acute treatment (generally within 3 weeks) allows ligaments to be reconstructed/repaired before significant scarring, potentially preserving knee kinematics and reducing further meniscal or chondral damage.[40] However, arthrofibrosis remains a valid risk. A staged repair addresses extra-articular structures initially, with cruciate ligament reconstruction after regaining full movement, typically 6-8 weeks later.[40] Delayed reconstruction (generally after 3 weeks) focuses on improving knee ROM and avoids reconstructing/repairing structures that may heal on their own.[40] In general, a 10-14-day delay is sufficient to reduce swelling and enable safer arthroscopic evaluation, with MRI recommended if not initially performed, as it aids in identifying injury specifics.[43] Waiting up to 21 days allows for an effective repair of bony avulsions or soft-tissue ligament or tendon avulsions, along with arthroscopic cruciate ligament reconstruction if needed.[43]
RECONSTRUCTION VERSUS REPAIR
Typically, knee ligament injuries can only be potentially repaired if surgery is performed within 3 weeks of the injury (acutely).[40] Beyond this period, ligament reconstruction is favored, as the integrity and definition of the soft-tissue planes diminish over time. Research dating back many decades has shown poor outcomes from primary mid-substance ACL repair, establishing reconstruction as the preferred treatment.[10,46] While the PCL has some healing ability, most complete PCL tears co-occur with other ligament injuries, making reconstruction more effective. Biomechanical and clinical studies consistently show that reconstruction of both the PCL and PLC provides superior stability and function.[47,48] Stannard et al.[49] reported much lower failure rates for anatomic reconstruction (9%) compared to primary repair (37%) in PLC injuries, findings echoed by Levy et al.,[44] who found reconstruction yielded better stability, ROM, and return to pre-injury activity. For the PCL, studies indicate that double-bundle (DB) reconstruction closely restores native strength and kinematic control, offering better restraint against posterior tibial translation than single-bundle (SB) approaches.[50]
CHOICE OF GRAFTS
In multiligament knee reconstructions, graft selection involves autografts and allografts, tailored to injury complexity and patient factors such as age, activity level, and surgeon preference.[10] Autografts, favored for biological compatibility and lower immune rejection risk, include bone-patellar tendon-bone (BPTB), hamstring (HS), quadriceps tendon, and peroneus longus. BPTB is regarded as the gold standard due to its strength and fixation but may cause anterior knee pain or patellar fractures.[51] HS grafts have lower donor-site morbidity but can reduce knee flexion strength and have a higher re-rupture rate.[52] Quadriceps tendon grafts offer robust strength and flexibility but may cause anterior thigh pain.[40,51] Peroneus longus grafts, effective for ACL, PCL, and PLC reconstructions, are valuable in low-resource settings.[53,54] Allografts, such as Achilles and tibialis anterior tendons, eliminate donor-site morbidity and facilitate complex reconstructions but carry higher costs, delayed incorporation, and potential immune response.[40] They are preferred when autografts are unavailable or in extensive injuries. Table 2 summarizes the commonly used graft types, their applications, benefits, and limitations.[40,55] While randomized controlled trials provide high-quality evidence for graft choice and tensioning strategies, the limited sample sizes and lack of long-term follow-up in many studies reduce their generalizability. Observational studies contribute valuable insights but often lack control groups, which may introduce selection bias.
| Graft type | Applications | Benefits | Limitations |
|---|---|---|---|
| Hamstring tndons (gracilis and semitendinosus) autograft | ACL, PCL, PLC, PMC, sMCL | • Long grafts that can be quadrupled for increased diameter • Simple harvesting technique • Low risk of donor site complications |
• Requires soft-tissue fixation • May be too small in some patients • Harvesting can extend surgery time |
| Bone-patellar tendon-bone (BPTB) autograft | ACL, PCL | • Bone-to-bone fixation offers strong anchoring • Durable and thick graft |
• Rare complications like patella fractures or tendon rupture • Cannot be used if QT graft with a bone plug is taken from the same knee • Harvesting increases surgery duration |
| Quadriceps tendon (QT) autograft | ACL, PCL | • Bone-to-bone fixation at one end possible • Strong and robust graft • Minimal risk of complications at the donor site |
• Rare risks such as patella fractures or quadriceps ruptures • Not an option if BPTB graft with a bone plug is taken from the same knee • Harvesting increases surgery time |
| Peroneus longus tendon autograft | ACL, PCL, PLC | • Provides sufficient length and strength • Minimal donor site complications • Effective in low-resource settings |
• Risk of foot or ankle instability • May be less commonly used, requiring surgeon familiarity |
| Achilles tendon allograft | ACL, PCL, PLC | • Long and wide graft • Bone-to-bone fixation at one end • No donor site complications • Reduction in operation time |
• Risk of disease transmission • Costly and sometimes unavailable |
| Tibialis anterior allograft | ACL, PCL, PLC | • Sufficient graft length • No donor site complications • Reduces operating time |
• Requires soft-tissue fixation • Risk of disease transmission • Expensive and limited availability |
| BPTB allograft | ACL, PCL | • Offers the same benefits as BPTB autograft • No donor site complications • Reduces operating time |
• Risk of disease transmission • High cost and limited availability |
ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, PLC: Posterolateral corner, PMC: Posteromedial corner, sMCL: Superficial medial collateral ligament, BPTB: Bone-patellar tendon-bone, QT: Quadriceps tendon
SURGICAL RECONSTRUCTION IN MLKIS
When surgical reconstruction is indicated, various techniques have been proposed to best restore native stability and biomechanics. Some reconstruction techniques with evidence supported by the literature are listed below for MLKI injuries [Figure 9].
![Examples of reconstruction techniques of the four major knee ligament complexes in a multiligament knee injury. (a) Anterior cruciate ligament reconstruction (ACLR), (b) double bundle posterior cruciate ligament reconstruction (DB-PCLR), (c and d) posteromedial complex reconstruction (PMCR), superficial medial collateral ligament (PMCR), (e) posterolateral corner reconstruction (PLCR), (f) fibular collateral ligament reconstruction (FCLR). ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: Posteromedial bundle, POL: Posterior oblique ligament, FCL: Fibular collateral ligament, PFL: Popliteofibular ligament. (Reprinted from LaPrade et al., 2019, with permission).[67]](/content/115/2025/0/1/img/JASSM-62-2024-g009.png)
- Examples of reconstruction techniques of the four major knee ligament complexes in a multiligament knee injury. (a) Anterior cruciate ligament reconstruction (ACLR), (b) double bundle posterior cruciate ligament reconstruction (DB-PCLR), (c and d) posteromedial complex reconstruction (PMCR), superficial medial collateral ligament (PMCR), (e) posterolateral corner reconstruction (PLCR), (f) fibular collateral ligament reconstruction (FCLR). ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: Posteromedial bundle, POL: Posterior oblique ligament, FCL: Fibular collateral ligament, PFL: Popliteofibular ligament. (Reprinted from LaPrade et al., 2019, with permission).[67]
ACL RECONSTRUCTION (ACLR)
In MLKI cases, ACLR may be performed with an anatomical SB technique, usually using a BPTB graft, quadriceps, and HS autografts from either the ipsilateral or contralateral knee.[40] The ACL femoral attachment can be triangulated using key landmarks, including the lateral intercondylar ridge and cartilage margins, and the ACL tibial attachment is directly inline with the anterior horn of the lateral mensicus.[56] The tunnels for an anatomic ACLR should aim for these anatomic locations. If performed acutely or when autografts are unavailable, allografts are considered.[40] While some advocate for DB reconstruction, studies show similar outcomes with SB techniques.[57]
PCL RECONSTRUCTION (PCLR)
The PCL is the largest and strongest intra-articular ligament of the knee.[48] It consists of two codominant bundles: The anterolateral bundle (ALB) and the posteromedial bundle (PMB). The PCL femoral attachment is approximately twice the size of its tibial insertion.[48,58] SB-PCLR reconstructs only the ALB, while DB-PCLR restores both the ALB and PMB. Recent studies have shown that DB-PCLR techniques more effectively restore knee kinematics and result in less residual posterior translation compared to SB reconstruction.[40,59] Specifically, biomechanical studies show DB-PCLR achieves superior knee kinematics, rotational stability, and higher IKDC scores compared to SB-PCLR.[48,58] This technique is performed by creating separate femoral tunnels for each bundle at their anatomic attachments, allowing independent tension and fixation knee flexion angles (90° for ALB and 0° for PMB), that enhance overall knee stability.[60] The preferred surgical approach of LaPrade et al.[48] is a DBPCLR using an 11 mm Achilles tendon allograft for the ALB and a 7 mm tibialis anterior allograft for the PMB. In cases where allografts are unavailable or not preferred, the ALB can be reconstructed with a quadriceps tendon autograft including a bone plug, while the PMB is reconstructed using a semitendinosus autograft.[48]
PMC RECONSTRUCTION
The PMC of the knee comprises several structures, including the superficial and deep MCL, POL, semimembranosus tendon, and the posterior horn of the medial meniscus. The sMCL is the main medial stabilizer, with a femoral and two tibial attachments, while the deep MCL is a thickening of the joint capsule that adheres to the medial meniscus.[61] The POL has three fascial arms, with the central arm reinforcing the deep MCL and blending into the joint capsule and meniscal junction.[62] The semimembranosus tendon dynamically stabilizes this area through its multiple tibial attachments, including the anterior and direct arms, which connect near the proximal superficial MCL and medial tibial crest, respectively.[62] An anatomically based technique has been developed to reconstruct both the sMCL and the POL, utilizing two grafts and four tunnels. The sMCL is secured proximally in a tunnel at its femoral attachment and distally 6 cm from the tibiofemoral joint line with either a tunnel (for allografts) or with suture anchors placed at its tibial attachment (for HS autografts) to enhance medial knee stability.[63] The sMCL should also be fixated about 12 mm distal to the joint line with a suture anchor. The POL is reconstructed using closed socket tunnels at its native sites. The MCL is fixated in 20-30° of flexion, while the POL is fixated in full extension to avoid overconstraint the graft.[63]
PLC RECONSTRUCTION
The PLC consists of three primary stabilizers: the FCL, PLT, and PFL. The FCL attaches on the femur 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle and extends to the lateral aspect of the fibular head about 8 mm from the anterior aspect of the fibular head on the mid-lateral aspect of the fibular head. The PLT originates at the popliteal sulcus and anchors distally at the tibial cortex.[64] The PFL has two divisions, with the posterior and anterior divisions attaching 1.6 mm and 2.8 mm distal to the fibular styloid process tip, respectively.[3,65] LaPrade et al.[65] introduced a now-classic anatomical technique for reconstructing the PLC, utilizing a split Achilles tendon allograft to restore the three primary PLC structures. In LaPrade’s PLC reconstruction technique, the first graft is used to reconstruct the FCL and PFL. This graft is anchored to mimic the natural attachments of the FCL and PFL, providing essential lateral and rotational stability to the knee by reinforcing structures that resist varus and external rotational forces.[65] The second graft focuses on reconstructing the PLT, and this graft restores the tendon’s ability to stabilize the knee against excessive external rotation, working in conjunction with the FCL and PFL reconstructions to provide a balanced reconstruction that addresses the primary components of the PLC.[65,66] In a recent case series by LaPrade et al.,[67] there was no significant difference in the postoperative functional and objective outcomes scores between sports-related ACL- and PCL-based MLKIs, and this was primarily attributed to the DBPCLR technique, as well as modern rehabilitation principles.
TENSIONING SEQUENCE
A biomechanical study by Moatshe et al.[68] highlighted the importance of tensioning grafts in a specific order to prevent abnormal rotational forces. Kennedy et al.[50] demonstrated that DB PCLRs minimize graft forces when the ALB is tensioned at 90° flexion and the PMB in full extension. Moatshe et al.[68] and LaPrade et al.[4] propose a similar sequence for graft tensioning and fixation, emphasizing the importance of flexion angles to optimize biomechanical restoration. Both suggest starting with the PCL to restore the central pivot, fixating the ALB at 90° flexion and the PMB in full extension (0°). The ACL is fixated in full extension, followed by the PLC grafts, with the FCL fixated in the fibular head at 20° flexion, and the PFL and PLT fixated at 60° of flexion.[4,68] Finally, the sequence concludes with the posteromedial corner, including the MCL fixated at 20° flexion in neutral rotation and the POL fixated at 0° [Figure 10].[4,68]
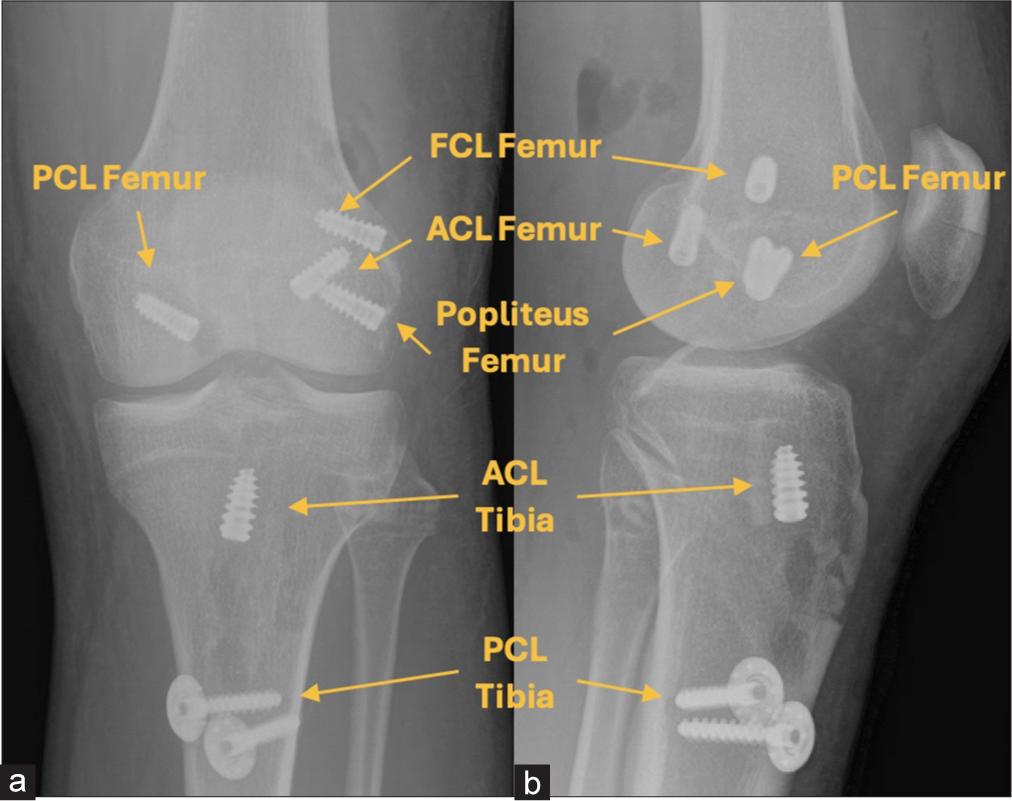
- (a) Anteroposterior and (b) lateral postoperative radiographs illustrating the reconstruction of the anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), fibular collateral ligament, popliteus tendon, and popliteofibular ligament. The ACL graft is secured with titanium screws at both the femoral and tibial attachments. Additional titanium screws anchor the anterolateral PCL bundle, fibular collateral ligament (FCL), and popliteus tendon at the femur. A post and spike washer are used to stabilize the anterolateral and posterolateral PCL bundles at the tibia, while bioabsorbable screws secure the posteromedial PCL bundle and posterolateral corner grafts at the fibular head and tibia (not shown).
AVOIDING TUNNEL CONVERGENCE
Tunnel converge is a complication that can be avoided with proper preoperative planning. On the medial femoral condyle, the sMCL tunnel should be aimed 40° in the axial and coronal planes and the POL tunnel 20° in the axial and coronal planes to avoid collision with the DB-PCL tunnels [Figure 11].[69]
![The femoral reconstruction tunnel for the superficial medial collateral ligament (sMCL) should be directed at a 40° angle anteriorly and proximally to avoid interference with the posterior cruciate ligament tunnels. With the patient positioned supine, the surgeon lowers their hand and guides the reamer 40° upward and toward the hip joint. Similarly, the femoral reconstruction tunnel for the posterior oblique ligament is oriented at a 20° angle proximally and anteriorly to prevent overlap with the sMCL tunnel. Angles “α” and “β” represent 40° and 20°, respectively, ensuring precise tunnel placement. The x line represents the x axis (medial to lateral), the z line represents the z axis (proximal to distal), and the y line represents the y axis (anterior to posterior). PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: Posteromedial bundle, POL: Posterior oblique ligament. (Reprinted from Moatshe et al., 2016, with permission).[69]](/content/115/2025/0/1/img/JASSM-62-2024-g011.png)
- The femoral reconstruction tunnel for the superficial medial collateral ligament (sMCL) should be directed at a 40° angle anteriorly and proximally to avoid interference with the posterior cruciate ligament tunnels. With the patient positioned supine, the surgeon lowers their hand and guides the reamer 40° upward and toward the hip joint. Similarly, the femoral reconstruction tunnel for the posterior oblique ligament is oriented at a 20° angle proximally and anteriorly to prevent overlap with the sMCL tunnel. Angles “α” and “β” represent 40° and 20°, respectively, ensuring precise tunnel placement. The x line represents the x axis (medial to lateral), the z line represents the z axis (proximal to distal), and the y line represents the y axis (anterior to posterior). PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: Posteromedial bundle, POL: Posterior oblique ligament. (Reprinted from Moatshe et al., 2016, with permission).[69]
Similarly, the FCL and PLT tunnels on the lateral femoral condyle should be aimed 35-40° anteriorly to avoid overlap with the ACL tunnel [Figure 12].[69] Angles exceeding 40° should be avoided as they may create elliptical tunnels, compromising graft fixation.[70] Preoperative planning and intraoperative fluoroscopy are critical tools for optimizing tunnel placement.[38]
![To avoid convergence with the anterior cruciate ligament tunnel, the fibular collateral ligament (FCL) tunnel should be angled 35° anteriorly. This alignment is achieved by lowering the surgeon’s hand and directing the reamer upward while the patient is in a supine position. The popliteus tendon tunnel is positioned parallel to the FCL tunnel, also at a 35° angle, to ensure it does not breach the intercondylar notch. The angle “α” represents 35° forward from the horizontal plane (x-axis), ensuring accurate and non-interfering tunnel placement. The x line represents the x axis (medial to lateral), the z line represents the z axis (proximal to distal), and the y line represents the y axis (anterior to posterior). PLT: Popliteus tendon, ACL: Anterior cruciate ligament. (Reprinted from Moatshe et al. 2016, with permission).[69]](/content/115/2025/0/1/img/JASSM-62-2024-g012.png)
- To avoid convergence with the anterior cruciate ligament tunnel, the fibular collateral ligament (FCL) tunnel should be angled 35° anteriorly. This alignment is achieved by lowering the surgeon’s hand and directing the reamer upward while the patient is in a supine position. The popliteus tendon tunnel is positioned parallel to the FCL tunnel, also at a 35° angle, to ensure it does not breach the intercondylar notch. The angle “α” represents 35° forward from the horizontal plane (x-axis), ensuring accurate and non-interfering tunnel placement. The x line represents the x axis (medial to lateral), the z line represents the z axis (proximal to distal), and the y line represents the y axis (anterior to posterior). PLT: Popliteus tendon, ACL: Anterior cruciate ligament. (Reprinted from Moatshe et al. 2016, with permission).[69]
In the proximal tibia, the POL tunnel should not be aimed directly at Gerdy’s tubercle, as this increases the risk of convergence with the PCL tunnel.[71] Instead, directing the POL tunnel 15 mm medial to Gerdy’s tubercle minimizes this risk [Figure 13]. To avoid collision between the sMCL and PCL tunnels, the sMCL tunnel should be angled 30° distally [Figure 14].[71]
![To prevent convergence between the posterior oblique ligament (POL) tunnel and the posterior cruciate ligament (PCL) tunnel (marked in green), the POL tunnel should be positioned 15 mm medial to Gerdy’s tubercle (highlighted in red) along the horizontal plane. Aiming directly at Gerdy’s tubercle (indicated in yellow) increases the likelihood of intersecting with the PCL tunnel. The anterior cruciate ligament tunnel is represented in blue, while the posterolateral corner tunnel is shown in purple. (a) A lateral view illustrates the POL tunnel exit, and (b) an axial view demonstrates the correct trajectory for the POL tunnel. L indicates lateral, and M indicates medial (Reprinted from Moatshe et al. 2016, with permission).[69]](/content/115/2025/0/1/img/JASSM-62-2024-g013.png)
- To prevent convergence between the posterior oblique ligament (POL) tunnel and the posterior cruciate ligament (PCL) tunnel (marked in green), the POL tunnel should be positioned 15 mm medial to Gerdy’s tubercle (highlighted in red) along the horizontal plane. Aiming directly at Gerdy’s tubercle (indicated in yellow) increases the likelihood of intersecting with the PCL tunnel. The anterior cruciate ligament tunnel is represented in blue, while the posterolateral corner tunnel is shown in purple. (a) A lateral view illustrates the POL tunnel exit, and (b) an axial view demonstrates the correct trajectory for the POL tunnel. L indicates lateral, and M indicates medial (Reprinted from Moatshe et al. 2016, with permission).[69]
![A three-dimensional model demonstrates the spatial relationship between the superficial medial collateral ligament (sMCL) tunnel (pink) and the posterior cruciate ligament tunnel (green). When the sMCL tunnel is positioned anterior to the fibular shaft in the horizontal plane, the two tunnels are in close proximity. To avoid convergence, the sMCL tunnel should be oriented transversely across the tibia, positioned ahead of the fibula, and angled 30° distally (light blue). The anterior cruciate ligament tunnel is shown in dark blue for reference (Reprinted from Moatshe et al. 2016, with permission).[69]](/content/115/2025/0/1/img/JASSM-62-2024-g014.png)
- A three-dimensional model demonstrates the spatial relationship between the superficial medial collateral ligament (sMCL) tunnel (pink) and the posterior cruciate ligament tunnel (green). When the sMCL tunnel is positioned anterior to the fibular shaft in the horizontal plane, the two tunnels are in close proximity. To avoid convergence, the sMCL tunnel should be oriented transversely across the tibia, positioned ahead of the fibula, and angled 30° distally (light blue). The anterior cruciate ligament tunnel is shown in dark blue for reference (Reprinted from Moatshe et al. 2016, with permission).[69]
REHABILITATION
In the early postoperative phase after MLKI surgery, rehabilitation prioritizes a delicate balance between protection, strengthening, and the gradual restoration of mobility, customized to the specific complexity of the multiligament injury.[4] Early single-stage surgical intervention allows for the timely initiation of ROM exercises, reducing the risk of arthrofibrosis and promoting joint health.[5] From the 1st postoperative day, cryotherapy is implemented to control pain and swelling.[4] Compression devices may also be used alongside cryotherapy to reduce swelling and improve early joint mobility. Baseline radiographs are obtained, and physical therapy begins immediately, focusing on patient education and patellar mobilization.[4] For patients with PCL injuries, passive ROM exercises are performed in a prone position to decrease stress on the graft.[5] Initially, ROM is restricted to 90° during the first 2 weeks and is gradually increased as recovery advances, with continued precautions to limit posterior tibial translation.[4]
Weightbearing is generally restricted following surgery, with patients remaining non-weightbearing for approximately 6 weeks.[72] During this period, patients transition from an immobilizer to a brace tailored to the type of injury. For cases involving PCL injuries, a dynamic PCL rebound brace may be introduced within 3-5-day post-surgery to enhance knee stability and facilitate controlled movement. Early quadricep activation is emphasized to minimize stiffness and improve recovery, starting with isometric exercises at shallow knee flexion angles and gradually progressing in intensity.[4] HS activation, however, is typically delayed for 8 weeks to safeguard tendon healing when applicable.[72] Specific protocols also vary based on the type of graft used. For example, allografts often require prolonged immobilization to reduce the risk of graft failure, whereas autografts may allow for earlier progression due to their higher tensile strength in the early postoperative period.[73]
Additional therapeutic modalities, such as neuromuscular electrical stimulation, can be employed to enhance muscle activation, mitigate atrophy, and maintain joint stability. Blood flow restriction therapy is another valuable tool, aiding in muscle strength improvement and bone density preservation during the non-weight bearing phase.[67] High-intensity interval training has been increasingly explored in the later stages of rehabilitation to improve cardiovascular fitness and functional recovery, especially in athletes. Functional proprioception exercises may also be incorporated in the later stages to restore balance and improve joint mechanics as weightbearing restrictions are lifted.
While early rehabilitation focuses on protecting the surgical site and promoting ROM, long-term success is often determined by the gradual restoration of strength, stability, and neuromuscular control. Potential complications such as graft failure, joint instability, or stiffness can arise if rehabilitation protocols are not strictly followed. Structured follow-up at 6-12 months is critical to assess functional outcomes and detect any issues requiring intervention.
As rehabilitation progresses, the program intensifies to build strength, stability, and cardiovascular fitness in a structured, symptom-tolerant manner. Return-to-sport protocols are individualized and based on objective measures, such as isokinetic strength testing, single-leg hop tests, and patient-reported outcome measures. Regarding long-term success rates, there is limited data specifically addressing outcomes of different rehabilitation strategies for MLKI patients. Physical performance is monitored at 3-4-month intervals to guide further progression and provide objective data for decisions regarding the return to activities or sports, aiming to achieve full recovery within 9-12 months.[4]
OUTCOMES
The evaluation of MLKI outcomes has historically been hindered by factors such as small study populations, inconsistent treatment protocols, and an underappreciation of the role of early postoperative rehabilitation. However, a recent study by LaPrade et al.[67] examined 194 athletes with low-velocity MLKIs, with a mean follow-up of 3.5 years. Reconstruction in this study involved anatomically and biomechanically validated techniques that were performed in a single-stage procedure, along with functional rehabilitation starting on the 1st postoperative day.[4] The results demonstrated improvements, including significant increases in Tegner activity levels (mean increase from 1 to 6), Lysholm scores (from 41 to 90), and a significant reduction in WOMAC scores (from 44 to 3). These positive outcomes were consistent across various ligament combinations and were comparable between acute and chronic cases.[67] These findings are consistent with the broader literature. For instance, a study published by Blokland et al.[74] reported that approximately 88.5% of patients returned to sports after MLKI, although only 23.1% were able to return to their pre-injury level of sport participation. Furthermore, 83.3% of patients successfully returned to work post-recovery, highlighting the variability in achieving full functional recovery. Similarly, studies by Hohmann et al.[75] and Sheth et al.[76] have reported favorable outcomes for MLKI reconstruction.
Furthermore, data from studies on traumatic knee dislocations further support these conclusions. For instance, Engebretsen et al.[77] studied 89 patients with knee dislocations and reported a median Lysholm score of 83 and a Tegner score of 5 at a minimum 2-year follow-up, although a significant proportion of patients developed grade II or higher Kellgren-Lawrence arthritis over time. The timeframe for return to sport also varies. A study by Borque et al.[78] has shown that some elite athletes resume play approximately 12.8-month post-surgery. Patient-reported outcomes also reveal variability. A systematic review by Everhart et al. noted that while many patients return to some level of activity, the rate of return to pre-injury levels remains lower.[79] Factors such as age, concomitant injuries, and adherence to rehabilitation protocols significantly impact recovery outcomes.[79]
These findings underscore the complexity of MLKIs and the challenges in achieving pre-injury activity levels. Individualized rehabilitation programs and realistic goal-setting are critical for optimizing functional outcomes and improving the likelihood of returning to pre-injury activity levels.
CONCLUSION
Managing MLKIs requires a precise diagnosis, surgical planning, and structured rehabilitation. Treatment choice depends on patient-specific factors, with surgical reconstruction typically providing superior stability and functionality. Timing of surgery and decisions on repair versus reconstruction are crucial to optimize outcomes and minimize complications like tunnel convergence. Tailored graft selection and advancements in PCL DB techniques improve knee kinematics. Early mobilization and progressive rehabilitation are a key to achieving long-term stability and favorable outcomes. Focusing on each element of care, from surgical intervention to rehabilitation, this approach supports long-term knee stability and function, enabling patients to achieve favorable outcomes.
Author contributions
BS, JEN, LVT, RFL: Concept and design of the study; BS, JEN: Literature review; BS, JEN: Analysis and interpretation of literature; All authors were involved in drafting of the article or revising, final approval, and all authors are accountable for all aspects.
Ethical approval
The Institutional Review Board approval is not required since it was a narrative review.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Conflicts of interest
Robert F. LaPrade reports a relationship with Ossur Americas that includes: consulting or advisory. Robert F. LaPrade reports a relationship with Smith and Nephew, Inc., that includes: consulting or advisory. Robert F. LaPrade reports a relationship with Linvatec Europe that includes: consulting or advisory. Robert F. LaPrade reports a relationship with Responsive Arthroscopy that includes: consulting or advisory. Robert F. LaPrade reports a relationship with Ossur Americas, Inc., that includes: funding grants. Robert F. LaPrade reports a relationship with Smith and Nephew, Inc., that includes: funding grants. Robert F. LaPrade reports a relationship with Arthroscopy Association of North America that includes: funding grants. Robert F. LaPrade reports a relationship with American Orthopaedic Society for Sports Medicine that includes: funding grants.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Classification of knee dislocations. Oper Tech Sports Med. 2003;11:193-8.
- [CrossRef] [Google Scholar]
- The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89:2000-10.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and biomechanics of the lateral side of the knee and surgical implications. Sports Med Arthrosc Rev. 2015;23:2-9.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple ligament anatomic-based reconstructions of the knee: State-ofthe-art. J Arthrosc Surg Sports Med. 2021;3:18-33.
- [CrossRef] [Google Scholar]
- Postoperative rehabilitation and return to sport following multiligament knee reconstruction. Arthrosc Sports Med Rehabil. 2022;4:e29-40.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular and nerve injury after knee dislocation: A systematic review. Clin Orthop Relat Res. 2014;472:2621-9.
- [CrossRef] [PubMed] [Google Scholar]
- Demographics and injuries associated with knee dislocation: A prospective review of 303 patients. Orthop J Sports Med. 2017;5:2325967117706521.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular injuries in knee dislocations: the role of physical examination in determining the need for arteriography. J Bone Joint Surg Am. 2004;86:910-5.
- [CrossRef] [Google Scholar]
- High-velocity knee dislocation with vascular injury. Treatment principles. Clin Sports Med. 2000;19:457-77.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of multiligament knee injury: State of the art. J ISAKOS. 2017;2:152-61.
- [CrossRef] [Google Scholar]
- Knee dislocation In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024.
- [PubMed] [Google Scholar]
- Multiple ligament knee injuries: Clinical practice guidelines. J Arthrosc Surg Sports Med. 2021;3:40-9.
- [CrossRef] [Google Scholar]
- Locked bucket-handle tears of both medial and lateral menisci with simultaneous anterior cruciate and medial collateral ligaments injury. BMJ Case Rep. 2011;2011:bcr0320114046.
- [CrossRef] [PubMed] [Google Scholar]
- The musculoskeletal examination In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods, The history, physical, and laboratory examinations. Boston: Butterworth Publishers, a division of Reed Publishing; 1990. Chapter: 164
- [Google Scholar]
- ACL and posterolateral corner injuries. Curr Rev Musculoskelet Med. 2020;13:123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Etiopathology and management of stiff knees: A current concept review. Indian J Orthop. 2021;55:276-84.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of knee ligament instabilities. Part I. The medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58:159-72.
- [CrossRef] [Google Scholar]
- Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58:173-9.
- [CrossRef] [Google Scholar]
- Physical examination of knee ligament injuries. J Am Acad Orthop Surg. 2017;25:280-7.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior cruciate ligament: Current concepts review. Arch Bone Jt Surg. 2018;6:8-18.
- [Google Scholar]
- The MR dimple sign in irreducible posterolateral knee dislocations. Skeletal Radiol. 2009;38:1111-4.
- [CrossRef] [PubMed] [Google Scholar]
- Physical examination and imaging of the acute multiple ligament knee injury. N Am J Sports Phys Ther. 2008;3:191-7.
- [Google Scholar]
- The diagnosis of PCL injury: Literature review and introduction of two novel tests. Iowa Orthop J. 2001;21:36-42.
- [Google Scholar]
- The management of injuries to the medial side of the knee. J Orthop Sports Phys Ther. 2012;42:221-33.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of the multiligament-injured knee. J Orthop Sports Phys Ther. 2012;42:234-42.
- [CrossRef] [PubMed] [Google Scholar]
- Difference in the joint space of the medial knee compartment between full extension and Rosenberg weight-bearing radiographs. Eur Radiol. 2022;32:1429-37.
- [CrossRef] [PubMed] [Google Scholar]
- Radiological assessment of lower limb alignment. EFORT Open Rev. 2021;6:487-94.
- [CrossRef] [PubMed] [Google Scholar]
- Mountain view of the patella. AJR Am J Roentgenol. 1981;136:53-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intraobserver and interobserver reliability of the kneeling technique of stress radiography for the evaluation of posterior knee laxity. Am J Sports Med. 2008;36:1571-6.
- [CrossRef] [PubMed] [Google Scholar]
- Fibular collateral ligament: Varus stress radiographic analysis using 3 different clinical techniques. Orthop J Sports Med. 2018;6:2325967118770170.
- [CrossRef] [PubMed] [Google Scholar]
- Stress radiography for the diagnosis of knee ligament injuries: A systematic review. Clin Orthop Relat Res. 2014;472:2644-57.
- [CrossRef] [PubMed] [Google Scholar]
- Stress radiography in the diagnosis of anterior cruciate ligament deficiency. Int Orthop. 1995;19:86-8.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral complex knee injuries: Magnetic resonance imaging with surgical correlation. Acta Radiol. 2005;46:297-305.
- [CrossRef] [PubMed] [Google Scholar]
- Location of bone bruises and other osseous injuries associated with acute grade III isolated and combined posterolateral knee injuries. Am J Sports Med. 2010;38:2502-8.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative MRI for the multiligament knee injury: What the surgeon needs to know. Curr Probl Diagn Radiol. 2020;49:188-98.
- [CrossRef] [PubMed] [Google Scholar]
- The value of accurate magnetic resonance characterization of posterior cruciate ligament tears in the setting of multiligament knee injury: Imaging features predictive of early repair vs reconstruction. Curr Probl Diagn Radiol. 2016;45:10-6.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of multiligamentous knee injury patterns with associated injuries presenting at a level I trauma center. J Orthop Trauma. 2013;27:226-31.
- [CrossRef] [PubMed] [Google Scholar]
- Strategies for preventing tunnel convergence in multiligament knee injury reconstructions. Indian J Orthop. 2024;58:1528-36.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of surgical repair or reconstruction of the cruciate ligaments versus nonsurgical treatment in patients with traumatic knee dislocations. Am J Sports Med. 2002;30:718-27.
- [CrossRef] [PubMed] [Google Scholar]
- Management of multiligament knee injuries. EFORT Open Rev. 2020;5:145-55.
- [CrossRef] [PubMed] [Google Scholar]
- Management of the multi-ligamentous injured knee: An evidence-based review. Ann Joint. 2019;4:21.
- [CrossRef] [Google Scholar]
- Surgical management of the multiple-ligament knee injury. Arthrosc Tech. 2018;7:e147-64.
- [CrossRef] [PubMed] [Google Scholar]
- Multiligamentous knee injuries-surgical treatment algorithm. N Am J Sports Phys Ther. 2008;3:198-203.
- [Google Scholar]
- Decision making in the multiligament-injured knee: An evidence-based systematic review. Arthroscopy. 2009;25:430-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and management strategies for multiligament knee injuries: A critical analysis review. JBJS Rev. 2016;4:e1.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95-100.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the outcomes of posterolateral corner knee injuries, part 1: Surgical treatment of acute injuries. Am J Sports Med. 2016;44:1336-42.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92:16-22.
- [CrossRef] [PubMed] [Google Scholar]
- The posterolateral corner of the knee: Repair versus reconstruction. Am J Sports Med. 2005;33:881-8.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior cruciate ligament graft fixation angles, part 2: biomechanical evaluation for anatomic double-bundle reconstruction. Am J Sports Med. 2014;42:2346-55.
- [CrossRef] [PubMed] [Google Scholar]
- Graft choices for anterior cruciate ligament reconstruction. Indian J Orthop. 2015;49:127-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior cruciate ligament reconstruction best practice: A review of graft choice. World J Orthop. 2014;5:23-9.
- [CrossRef] [PubMed] [Google Scholar]
- Peroneus longus autograft can be recommended as a superior graft to hamstring tendon in single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:3552-9.
- [CrossRef] [PubMed] [Google Scholar]
- Advances and trends in multiligament injuries of the knee relevant to low-resource settings. J Arthrosc Surg Sports Med. 2020;1:118-125.
- [CrossRef] [Google Scholar]
- Graft selection in surgicalreconstruction of the multiple-ligament-injured knee. Oper Techn Sports Med. 2003;11:218-25.
- [CrossRef] [Google Scholar]
- Anatomy of the anterior cruciate ligament and the common autograft specimens for anterior cruciate ligament reconstruction. Ann Jt. 2023;8:28.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of double-vs single-bundle anterior cruciate ligament reconstruction: A systematic review of randomized control trials. Scand J Med Sci Sports. 2013;23:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94:1936-45.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of single-bundle and double-bundle isolated posterior cruciate ligament reconstruction with allograft: A prospective, randomized study. Arthroscopy. 2014;30:695-700.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic posterior cruciate ligament reconstruction: State of the Art. J ISAKOS. 2016;1:292-302.
- [CrossRef] [Google Scholar]
- Posteromedial corner knee injuries: Diagnosis, management, and outcomes: A critical analysis review. JBJS Rev. 2017;5:e4.
- [CrossRef] [PubMed] [Google Scholar]
- Superficial medial collateral ligament anatomic augmented repair versus anatomic reconstruction: An in vitro biomechanical analysis. Am J Sports Med. 2013;41:2858-66.
- [CrossRef] [PubMed] [Google Scholar]
- An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38:339-47.
- [CrossRef] [PubMed] [Google Scholar]
- The posterolateral attachments of the knee: A qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31:854-60.
- [Google Scholar]
- An analysis of an anatomical posterolateral knee reconstruction: An in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32:1405-14.
- [CrossRef] [PubMed] [Google Scholar]
- Improving outcomes for posterolateral knee injuries. J Orthop Res. 2014;32:485-91.
- [CrossRef] [PubMed] [Google Scholar]
- Single-stage multiple-ligament knee reconstructions for sports-related injuries: Outcomes in 194 patients. Am J Sports Med. 2019;47:2563-71.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of graft tensioning sequence on tibiofemoral orientation during bicruciate and posterolateral corner knee ligament reconstruction: A biomechanical study. Am J Sports Med. 2018;46:1863-9.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple ligament reconstruction femoral tunnels: Intertunnel relationships and guidelines to avoid convergence. Am J Sports Med. 2017;45:563-9.
- [CrossRef] [PubMed] [Google Scholar]
- Tunnel convergence in combined anterior cruciate ligament and posterolateral corner reconstruction. Arthroscopy. 2006;22:193-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intertunnel relationships in the tibia during reconstruction of multiple knee ligaments: How to avoid tunnel convergence. Am J Sports Med. 2016;44:2864-9.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of functional brace forces for posterior cruciate ligament injuries on the knee joint: An in vivo investigation. Knee Surg Sports Traumatol Arthrosc. 2015;23:3070-6.
- [CrossRef] [PubMed] [Google Scholar]
- A modern-day algorithm for the treatment of multi-ligament knee injuries. Indian J Orthop. 2024;58:1566-78.
- [CrossRef] [PubMed] [Google Scholar]
- Low rate of return to pre-injury level of sports after multi-ligament knee injury-Functional outcomes after MLKI. Knee. 2021;33:65-72.
- [CrossRef] [PubMed] [Google Scholar]
- Early or delayed reconstruction in multi-ligament knee injuries: A systematic review and meta-analysis. Knee. 2017;24:909-16.
- [CrossRef] [PubMed] [Google Scholar]
- Early surgery of multiligament knee injuries may yield better results than delayed surgery: A systematic review. J ISAKOS. 2019;4:26-32.
- [CrossRef] [Google Scholar]
- Outcome after knee dislocations: A 2-9 years follow-up of 85 consecutive patients. Knee Surg Sports Traumatol Arthrosc. 2009;17:1013-26.
- [CrossRef] [PubMed] [Google Scholar]
- High return to play rate following treatment of multiple-ligament knee injuries in 136 elite athletes. Knee Surg Sports Traumatol Arthrosc. 2022;30:3393-401.
- [CrossRef] [PubMed] [Google Scholar]
- Return to work or sport after multiligament knee injury: A systematic review of 21 studies and 524 patients. Arthroscopy. 2018;34:1708-16.
- [CrossRef] [PubMed] [Google Scholar]







