Translate this page into:
Multiple ligament anatomic-based reconstructions of the knee: State- of-the-art
*Corresponding author: Robert F. LaPrade, Complex Knee and Sports Medicine, Twin Cities Orthopedics, 4010 W 65th St, Edina, MN 55423, United States. laprademdphd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: LaPrade RF, Floyd ER, Carlson GB, Moatshe G, Chahla J, Monson JK. Multiple ligament anatomic-based reconstructions of the knee: State of the art. J Arthrosc Surg Sports Med 2022;3:18-33.
Abstract
Multiple knee ligament injuries are defined as a disruption of any combination of the four main ligament complexes; the cruciate ligaments, posterolateral corner, and posteromedial corner. Evaluation requires consideration of the entire clinical picture, including injury to associated structures, directions and degree of instability, neurovascular compromise and appropriate imaging, and physical examination. Reconstruction is favored over repair and anatomic- based reconstruction techniques have been validated to restore the native biomechanics of the knee and lead to successful patient-reported and objective outcomes. Anatomic-based reconstruction of many knee ligaments simultaneously requires precise knowledge of the relevant anatomical landmarks, careful planning of reconstruction tunnel positions, and orientations to avoid tunnel convergence, and employment of immediate early motion in the post-operative rehabilitation regimen to provide the patient the best chance for relatively normal use of the affected limb.
Keywords
Multiple ligament reconstructions
Posterolateral corner
Posteromedial corner
Posterior cruciate ligament
Anterior cruciate ligament
INTRODUCTION
Historically, several controversies dominated the surgical management of multiple ligament injuries to the knee (MLKIs), namely “whether, when, and how?” Consideration of whether to operate, when to operate, if to repair or reconstruct the ligaments, and if so how to reconstruct the ligaments, prompted management of MLKIs to evolve from casting and immobilization to acute, single-stage, anatomical reconstruction of the knee ligaments over the past two to three decades. These injuries may present in the presence or absence of a frank knee dislocation, which usually involve a disruption of both cruciate ligaments according to the classification system created by Schenck[1-3] [Table 1].
| Schenck KD classification | Description of Injury: ACL, PCL, MCL, FCL |
|---|---|
| KD-I | Multiligament injury with (1) cruciate ligament injury (ACL or PCL) |
| KD-II | (2) Cruciate Ligaments involved (ACL and PCL) |
| KD-III-M | Injury to (3) ligaments: both ACL and PCL with additional medial injury (MCL) |
| KD-III-L | Injury to (3) ligaments: both ACL and PCL with additional lateral injury (fibular collateral ligament) |
| KD-IV | ACL + PCL and MCL + FCL injury (both cruciates and both collaterals, (4) ligaments total) |
| KD-V | Same as KD-IV but with peri-articular fracture (4) ligaments total |
KD: Knee dislocation, ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, MCL: Medial collateral ligament, FCL: Fibular collateral ligament
Depending on the force and direction of the inciting mechanism, MLKIs can encompass a wide range of injuries, including to one or both cruciate ligaments, posterolateral corner (PLC) and posteromedial corner (PMC) structures, capsular, vascular, nervous, and associated tendinous structures. The primary static stabilizers of the PLC are the fibular (lateral) collateral ligament (FCL), popliteofibular ligament (PFL), and popliteus tendon (PLT), while the medial-side stabilizers are the superficial medial collateral ligament (sMCL) and posterior oblique ligament (POL).[4-9] Damage also frequently encompasses the biceps femoris (BF) tendon, the menisci, the common peroneal nerve (CPN), and the joint capsule, including the meniscotibial and meniscofemoral portions of the deep MCL.[5,6,10]
Advanced Trauma and Life Support considerations are initially paramount in high energy injuries because up to 27% of high-energy knee dislocations present with concomitant life-threatening injuries.[3,8,11] Diagnosis must involve a thorough physical examination supplemented with stress radiography and magnetic resonance imaging (MRI). Early surgical management of multiple ligament injuries is now the preferred standard of care.[5,6,12,13] In this review, we will discuss the etiology, diagnosis, management, rehabilitation, and outcomes of surgical treatment of MLKIs.
INCIDENCE AND ETIOLOGY
MLKIs can be caused by high-energy trauma, low-energy sporting events, and ultra-low velocity mechanisms seen in the morbidly obese.[4,5,8] Studies have reported that high-energy etiologies make up around 50% of these injuries, and sports accidents account for 47–49%.[8,14] High grade PLC and PCL tears are often associated with concomitant ligament injuries; indeed, isolated tears of the PCL are reported to account for only 6.5% of acute traumatic knee injuries, while 52–85% of PCL injuries involve multiple ligaments.[15,16] Similarly, only 13–28% of Grade III (complete) PLC injuries occur in isolation, while the majority involve other ligaments, most commonly the ACL.[6,16-19] Isolated ACL injuries are more common, but it has been reported that 39% of ACL injuries presenting with hemarthrosis are part of multiple ligament injuries, most often ACL and MCL.[16,20-22] Medial-sided injuries constitute a majority of multiligament injuries overall (41–52%), compared to lateral-sided injuries (28%), and bicruciate injuries without collateral ligament involvement are uncommon (~5%).[23]
EVALUATION OF MULTILIGAMENT KNEE INJURIES
Physical examination
After ascertaining neurovascular status, an assessment of the patient with an MLKI should make use of a thorough physical examination supplemented with stress radiography and advanced imaging[5] [Figure 1]. Physical examination should include Lachman testing, posterior, posterolateral and anteromedial drawer tests, varus and valgus stress testing, along with the dial test, observation for pivot- shifting, and measurement of heel height. Acute patients with hemarthrosis may localize pain poorly and diffusely.[19] A varus thrust gait may develop in some patients with chronic PLC injuries due to dynamic destabilization of the lateral tibiofemoral articulation.[24] CPN injuries often present first with diminished dorsal foot sensation and loss of extensor hallucis longus motor function.
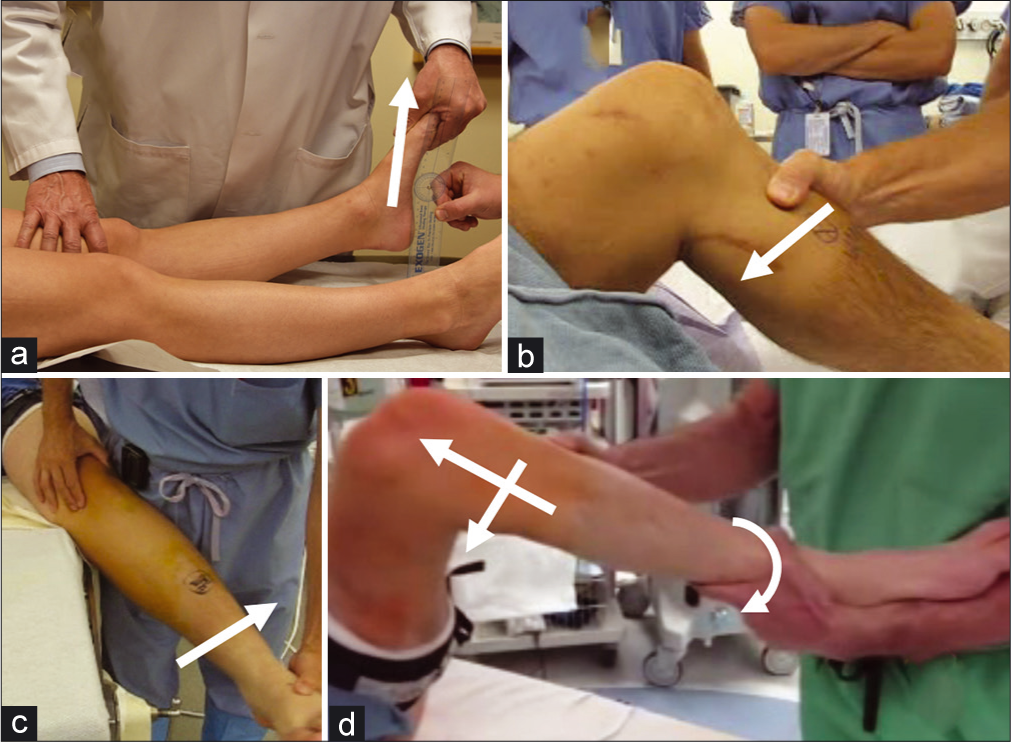
- Some Physical Examination Maneuvers Useful in the evaluation of Multiple Ligament Injuries of the Knee. (a) The external rotation recurvatum test compares heel height for each leg. A significant increase on the injured side (>3 cm) compared to the uninjured side is strongly suggestive of combined posterolateral corner (PLC) and anterior cruciate ligament (ACL injury). (b) The posterior drawer test demonstrates increased posterior tibial translation in the absence of a functioning PCL. (c) Valgus stress testing is positive when a significant side-to-side difference in medial-sided gapping is present relative to the uninjured leg, indicating disruption to the superficial medial collateral ligament and/or posterior oblique ligament. (d) The Pivot-shift phenomenon is observed as a sudden jerk on application of internal rotation and valgus force on the knee while flexing the hip. This is due to the action of the iliotibial band changing from extension to flexion at >30° of flexion. The jerking motion is really a sudden subluxation- anterior translation of the tibia due to ACL deficiency.
All examinations must be performed bilaterally to account for physiologic laxity; positive findings are relative to increased side-to-side difference (SSD) from the unaffected extremity to the injured side. Varus and valgus stress exams should be performed at 20–30° and in full extension, and the dial test observed at 30° and 90°. At 30°, a 10° SSD in external rotation on the dial test is suggestive of a PMC or PLC injury. A positive dial test at 90° may be indicative of PLC and combined PCL or a severe PMC injury.[25-28] To differentiate between a PMC or PLC injury, one should observe for anteromedial versus posterolateral knee rotation or valgus versus varus stress increases.[8,19,29,30]
Stress radiography
Varus, valgus, and posterior kneeling stress radiographs provide a method to objectively quantify ligament deficiency with dependable accuracy between clinicians [Figure 2]. Valgus stress radiographs have been validated for the detection of an isolated sMCL injury with a SSD of 1.7 mm of gapping in extension (or 3.2 mm gapping at 20° of flexion) and detection of a complete medial knee injury with 6.5 mm of valgus opening in extension (or 9.8 mm gapping at 20° of flexion).[31] [Table 2]. Varus stress radiography is used to detect an isolated FCL tear or more extensive PLC tear with 2.2 mm or >4.0 mm varus gapping, respectively.[7,32] Kneeling posterior stress radiography [Figure 3] has also been found to accurately measure partial, complete, or combined PCL injury with a SSD in posterior tibial translation of <8 mm, 8–11 mm, or >12 mm, respectively.[33] Stress radiographs are a useful, fast and cost-effective tool, although they may be limited by pain in the acute setting. In the chronic setting, Kane et al. reported an increased diagnostic efficacy for varus stress radiography over MRI in diagnosing complete FCL injuries; it is thought that some ligaments may heal in an elongated position and become nonfunctional without being visibly torn.[34]
| Extension Gapping | Flexion Gapping | Kneeling | |
|---|---|---|---|
| Isolated MCL, Valgus | 1.7 mm | 3.2 mm at 20° | N/A |
| Complete medial side (MCL+posterior oblique ligament), Valgus | 6.5 mm | 9.8 mm at 20° | N/A |
| PCL, Posterior Stress | N/A | N/A | Partial: 0–8 mm Complete: 8–11 mm Combined (with posterolateral or posteromedial corner): >12 mm |
| Isolated FCL, Varus | N/A | 2.2 mm at 20° | N/A |
| Complete PLC, Varus | N/A | >4 mm at 20° | N/A |
MCL: Medial collateral ligament, POL: Posterior oblique ligament, PLC: Posterolateral corner, FCL: Fibular collateral ligament, PCL: Posterior cruciate ligament
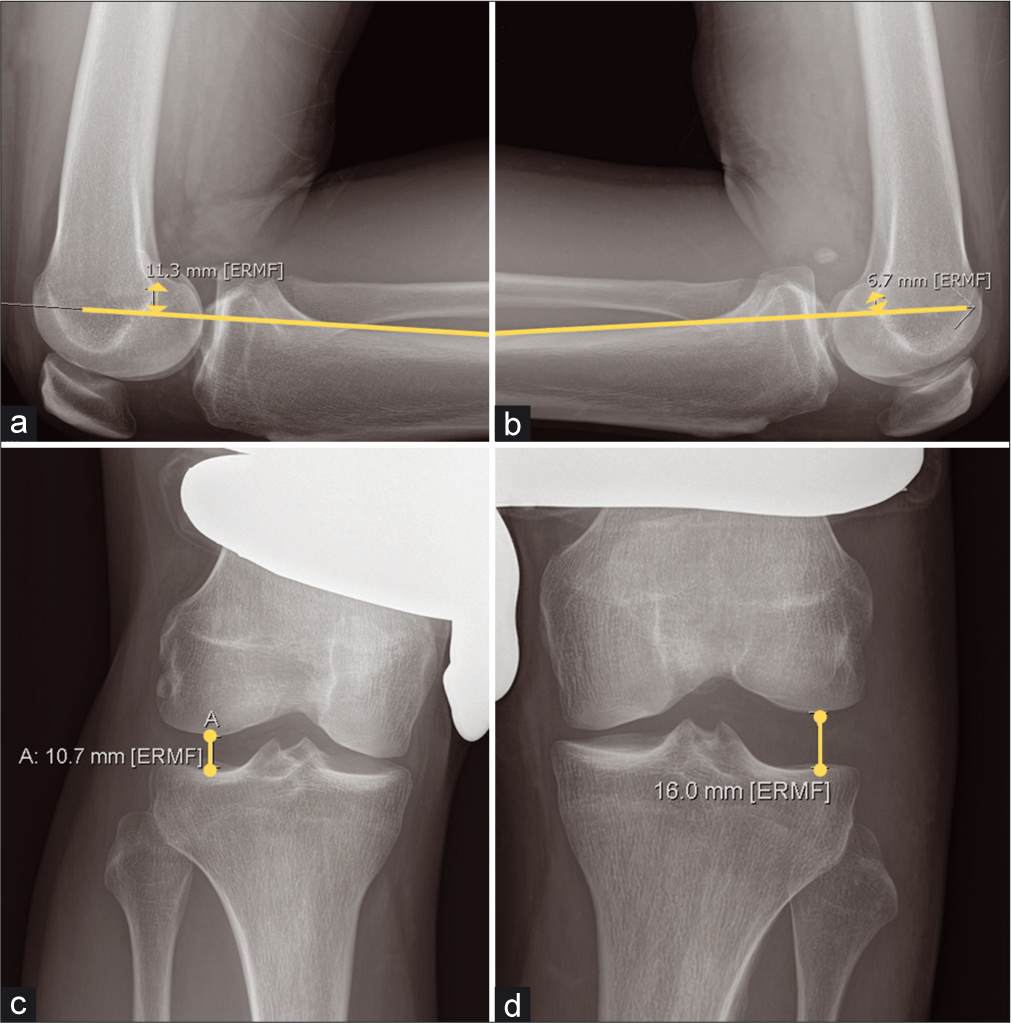
- Stress Radiographs: (a and b): Kneeling Posterior Stress Radiographs are an essential tool in evaluating posterior cruciate ligament (PCL) laxity. PCL deficiency results in greater posterior tibial translation on the injured side when the patient kneels upon the application of a posteriorly directed force, such as the patient kneeling with the proximal tibial joint line on the edge of a table or specially designed apparatus. A side-to-side difference (SSD) of 0-8 mm is suggestive of a partial PCL tear. A SSD of 8-11 mm is suggestive of a complete tear, while a SSD of >12 mm may indicate a combined injury, such as to the posterolateral or posteromedial corner in addition to PCL disruption. The SSD between (a and b) is 4.6. (c and d): Varus stress radiographs are validated to detect differences in valgus gapping indicative of fibular collateral ligament (FCL) or posterolateral corner (PLC) injury. A SSD of 2.2–4 mm in varus gapping is suggestive of an isolated grade III (complete) FCL tear, while >4.0 mm in SSD of varus gapping is indicative of a complete PLC injury.
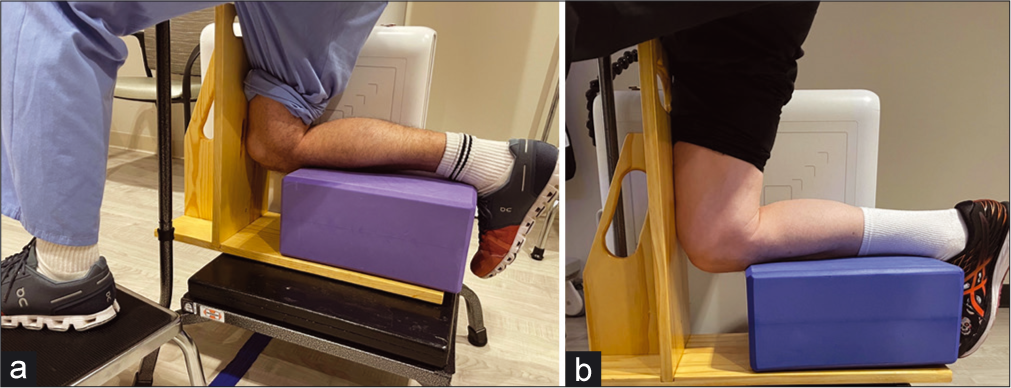
- Setup for kneeing Posterior Stress Radiographs: Posterior stress radiographs are an important tool for determining the integrity of the posterior cruciate ligament (PCL) in patients presenting with multiple ligament injuries. In chronic patients with an injury to the PCL, kneeling posterior stress radiographs can also determine whether the PCL healed in an attenuated position, rendering it nonfunctional. (a) Radiographs are taken by having the patient kneel on the injured leg, with the contralateral leg planted forwards, out of the way of the X-ray shot. The patient should have a bar or surface to hold for stability. (b): The patient’s kneeling leg is flexed, with the lower leg on a foam block and the tibial plateau just at the edge of the block. The patient’s weight falls through the femur and tests the integrity of the PCL. The radiographic difference is compared to the uninjured side to determine the side-to-side difference.
Advanced imaging
Diagnosis in the acute setting (<3 weeks) may be confounded by hemarthrosis and a physical examination limited by pain. MRI is almost universally ordered for MLKIs, with close to 100% sensitivity in acute MLKIs.[34,35] Geeslin and LaPrade reported MRI-lucent bone bruises in 80% of PLC injuries, mostly in the anteromedial femoral condyle, and should be considered evidence of a PLC injury until proven otherwise[36] [Figure 4]. Lateral femoral condyle and posterior lateral tibial plateau bruises are frequently associated with ACL injuries, and acute MCL injuries may be observed with a “wave- sign.”[37] Computed tomography (CT) arthrography is an alternative when MRI is not possible.
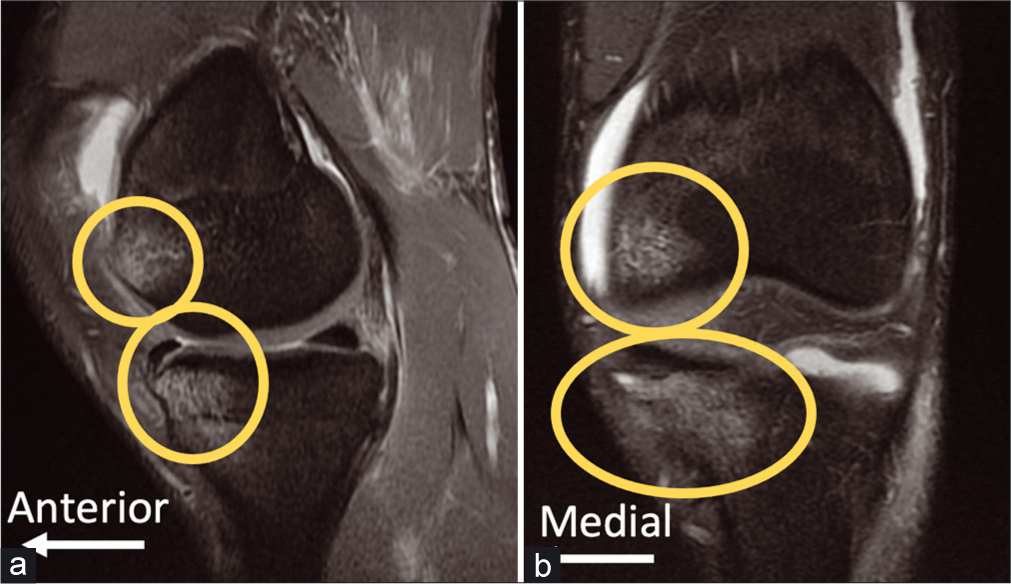
- Bone Bruises in Posterolateral Corner (PLC) Injuries are commonly observed on magnetic resonance imaging (MRI), and should be taken as evidence of PLC injuries until proven otherwise. The images above are sagittal (a) and coronal (b) fat- suppressed proton-dense T2-weighted MRI views of a left knee with an isolated PLC injury. Bone bruises of the medial compartment are thought to be common in PLC injuries due to the mechanism of hyperextension, frequently with a varus component. Medial compartment tibial plateau fractures are also common in PLC injuries, both phenomenon related to varus moment causing impaction in the medial compartment.
OPERATIVE OR NON-OPERATIVE TREATMENT
A consensus statement from the first ISAKOS meeting in 1995 indicated that single-stage, arthroscopic surgery for multiple ligament injuries should be attempted, and that the contemporary practice of casting and immobilization achieved inadequate results.[38] Over the past three decades, systematic reviews and meta-analyses have demonstrated that outcomes are improved significantly by acute surgical intervention, and by immediate post- operative commencement of passive range of motion (ROM).[1,6,12,13,39,40] A systematic review by Peskun and Whelan (2011) examined 35 studies and reported better results with early surgical management than non-operative management.[41] Dedmond and Almekinders performed a meta-analysis with 206 knees and reported that Lysholm scores improved to 85.2 in the operative group with a mean ROM of 123° versus 66.5 for the non-surgical group with a mean ROM of 108°.[42] Richter et al. described similar results (Lysholm 78.3 for surgical vs. 64.8 for non-surgical treatment), and additionally found that post-operative functional rehabilitation was the most significant prognostic factor overall, with a return to sports of 63% versus 39% for post-operative immobilization.[40]
ACUTE VERSUS DELAYED MANAGEMENT
Definitive treatment and optimal timing for addressing MLKIs have been historically controversial.[43,44] A systematic review by Mook et al. reported lower risk of additional treatment for joint stiffness with delayed or staged procedures compared to acute treatment, and noted that early rehabilitation in acute treatment was not associated with increased ligament laxity.[45] However, recent clinical outcome studies and systematic reviews favor acute surgical management with anatomic reconstructions.[6,12,13,39,41,45-47] It has also been reported that for acute MLKI injuries, early postoperative ROM reduces arthrofibrosis.[1,8,45] More recently, a meta-analysis by Hohmann et al. (2017) and systematic review by Sheth et al. (2019) concluded that early surgical intervention in MLKIs provides better clinical and functional outcomes according to existing literature.[12,13] Recent expert opinion International Consensus statements using modified Delphi processes for the PLC (2019) and PMC (2020) also favor anatomic reconstruction of both areas in the acute setting (2–3 weeks from injury) with early ROM protocols starting on post-operative day 1.[48,49]
Acute management of multiligament injuries
Various authors have proposed guidelines for the initial management of knee dislocations in the acute setting.[3,8,11] Damage to the popliteal artery is of paramount concern for traumatic knee injuries, with up to a 7% prevalence in dislocated knees.[3,11] Vascular status is monitored through physical examination, dorsalis pedis, and posterior tibial artery pulses. Selective arteriography is appropriate for pain, pallor, pulselessness, poikilothermia or an ankle-brachial index (ABI) of <0.9.[11] A normal physical exam and an ABI >0.9 have been reported to yield 100% sensitivity and a 100% negative predictive value for ruling out vascular injury.[50,51] Serial neurovascular checks for 48 h with a low threshold for pressure measurements and fasciotomy should be used to monitor for compartment syndrome.[3] There is only a 6–8 h window to restore perfusion and prevent amputation if blood flow is restricted.[11,52,53]
When faced with an open knee dislocation or popliteal artery injury, external fixation spanning the knee joint is appropriate to hold the knee reduced during healing of revascularization grafts or ruling-out of infection (at least 7 days).[3,8,43] Immobilization in 20° of flexion is preferred.[3,8,54] If infection risk is low and vascular integrity has been confirmed, reconstruction can proceed in 1–2 weeks after removal of the external fixator, otherwise waiting 2–6 weeks for healing of vascular grafts may be necessary.[3] The knee is placed in a hinged brace until surgery to avoid stiffness and protect the pin sites.[8]
CPN injuries are far more common than tibial nerve injuries in the setting of multiple ligament injuries, particularly in the presence of a PLC injury.[3,8,55] PLC injury has been reported to dramatically increase the odds of both popliteal artery and CPN injury.[8] Assessment of CPN function in the post-anesthesia care unit can be complicated by the administration of nerve blocks to the lower extremity, making it impossible to assess motor function until termination of the effects of the blocking procedure. A systematic review by Medina et al. and a study of 106 knees by Becker et al. both reported an incidence of 25% for CPN injury associated with MLKIs.[56,57]
Chronic management of multiple ligament injuries
Chronic injuries in this context refer to those presenting at >6 weeks from the inciting event. Multiple ligament injuries are frequently misdiagnosed at initial presentation or missed in the context of more life-threatening traumatic injuries. PLC tears may be missed in up to 72% of patients at initial presentation, and it has been reported that only 50% are correctly diagnosed at the time of referral to a specialist clinic.[58] Surgical management of delayed diagnosis of these injuries is more difficult because of the accumulation of scar tissue, which is of particular concern around the CPN where the nerve can become indistinguishable from the tissue of a torn BF tendon. CPN neurolysis, including 5–7 mm of release of the peroneus longus fascia overlying the nerve, is usually performed to minimize trauma to the nerve caused by swelling and stretching.[59]
Additional considerations to MLKIs
In addition to neurovascular compromise, injuries to multiple knee ligaments are frequently associated with cartilage lesions, meniscus tears, and intra- and periarticular fractures.[3,56,57,60] Several authors have reported rates of meniscal injury associated with MLKIs, including Richter (15%), Krych (55%), and Moatshe (37.3%).[8,40,60] In general, the authors recommend addressing all concomitant bone, ligament, meniscus, and cartilage damage in a single-stage procedure.[61]
An exception to this guideline is in cases of varus malalignment in patients with chronic PLC injuries [Figure 5]. Opening wedge proximal tibial osteotomy (PTO) has been used successfully to correct varus deformity in advance of PLC and multiligament reconstruction in the chronic setting.[62,63] Consideration may also be given to simultaneous sagittal plane correction, because posterior tibial slope has a profound effect on antero-posterior translational stability of the tibia.[64,65] Ligament reconstruction is then performed as a second stage after healing of the osteotomy, usually around 6–8 months.[62]
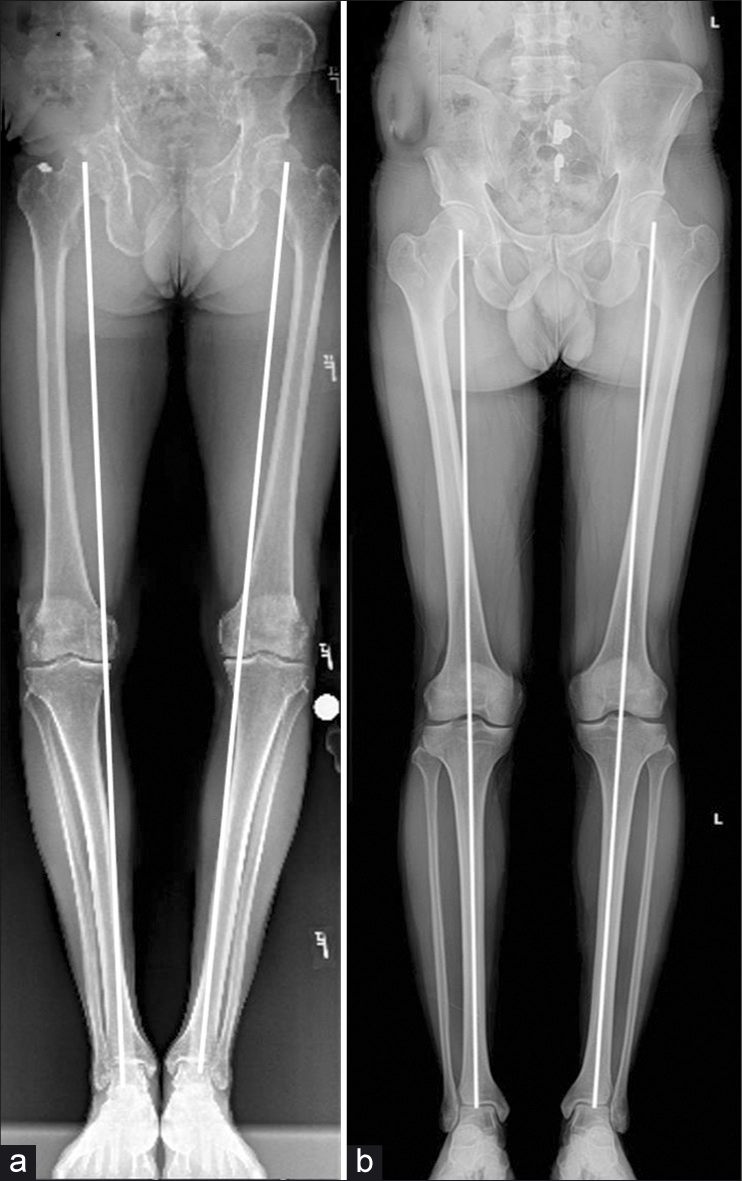
- Varus Alignment (a) is not tolerated by posterolateral corner reconstruction grafts and must be addressed prior to MLKI. Alignment is determined by drawing a line from the center of the femoral head to the midpoint of the dome of the talus. If the line passes through the center of the tibial spines, the patient is in neutral alignment. (b) If the lines traverses medial to the apex of the medial tibial spine (eminence), the patient is in varus alignment. Opening- wedge proximal tibial osteotomy is often chosen to address this deformity, and is one case where multiple ligament reconstructions of the knee should be staged.
REPAIR VERSUS RECONSTRUCTION OF LIGAMENTS
Studies dating back as far as the 1970’s have attested poor results from primary repair of the ACL, for which reconstruction is the mainstay of treatment.[8,66,67] Treatment of the PCL has been more controversial due to a greater ability of the PCL to heal after injury; however, most complete PCL tears occur concomitantly with other ligament injuries.[68,69] For complete PCL tears and PLC injuries, biomechanical studies and clinical outcomes have reported that greater stability and functional outcomes are achieved through reconstruction of the PCL and PLC.[6,10,18,19,68,70-75] Stannard et al. (2005) reported failure rates of 37% and 9% for primary repair and anatomic reconstruction techniques, respectively, in a cohort of 57 knees with PLC injuries at 2-year follow-up.[76] In their 2009 systematic review, Levy et al. reported a higher failure rate for primary repair versus reconstruction in complete PLC injuries (37% vs. 9%, respectively, identical to the Stannard study), and improved stability, ROM, and return to pre-injury activity level for reconstruction of the cruciate ligaments in MLKIs when compared with primary repair.[39] Geeslin et al. (2015) reported the results of a systematic review of acute PLC injuries; using objective criteria consisting of varus stress examinations and radiographs, the authors found a 9% failure rate for reconstructions versus a 19% failure rate for primary repair in the acute setting.[6] In a counterpart study for the chronic setting, Moulton et al. (2015) found that reconstruction of PLC injuries in the chronic setting led to a failure rate of 10%.[75] Most PLC injuries are combined; Geeslin and LaPrade (2010) found only 28% of PLC injuries were isolated.[28,36]
In consideration of the PCL, biomechanical studies by Kennedy et al. (2013) and Wijdicks et al. reported that both the anterolateral bundle (ALB) and posteromedial bundle (PMB) are co-dominant, having a synergistic effect in their restraint of posterior tibial translation. The authors determined robotically that a double-bundle reconstruction more effectively approximates native PCL restraint to posterior tibial translation and internal rotation.[77,78] The following year, Kennedy et al. (2014) reported another pair of biomechanical studies comparing double-bundle and single-bundle reconstruction. They found the single bundle reconstruction had an average 4.1 mm of residual laxity to posterior tibial translation compared to 1.5 mm of residual laxity in the double-bundle model. These data indicate that double-bundle posterior cruciate ligament reconstructions better restore native biomechanical strength and kinematic restraint to posterior tibial translation.[79,80]
GRAFT CHOICE
For the ACL, a bone-patellar-tendon bone autograft is recommended in most active, skeletally mature individuals.[81,82] Double-bundle PCL reconstruction has been shown to restore the native biomechanics of the knee better than single-bundle reconstructions.[5,68] The authors use an 11 mm wide Achilles tendon allograft with a proximal 11 × 20 mm bone block for the ALB, and a 7 mm tibialis anterior allograft, whip-stitched at each end, for the PMB.[68] Other graft options for a DB PCLR are using a quadriceps tendon autograft for the ALB and a hamstring autograft for the PMB. All three PLC structures, the FCL, PLT, and PFL, are reconstructed with a split Achilles tendon allograft, each with a proximal 9 × 20 mm bone plug and tubularized distally, as detailed in the anatomic PLC reconstruction method detailed by LaPrade et al.[72] Other authors have used hamstring autografts to perform anatomic PLC reconstructions.[83] On the medial side, two separate tibialis anterior allografts, or ipsilateral hamstring autografts, are used to reconstruct the POL and the sMCL, with average graft lengths of 16 cm for the sMCL and 12 cm for the POL, whip-stitched at each end.[84,85]
RELEVANT ANATOMY: IMPORTANT LANDMARKS
A precise knowledge of relevant landmarks for reconstruction is indispensable for addressing multiple ligament injuries [Table 3]. On the distal medial femur, the adductor magnus tendon is an essential guidepost to finding the relevant anatomy. The tendon attaches just superior to the adductor tubercle (AT), and the medial epicondyle (ME) can be identified 12.6 mm distal and 8.3 mm anterior to the AT.[86] From there, the sMCL attachment site is located in a bony depression 3.2 mm proximal and 4.8 mm posterior to the ME[84] [Figure 6a]. The central arm of the POL attaches to the femur 7.7 mm distal and 2.9 mm anterior to the gastrocnemius tubercle, which is located just distal and anterior to the attachment of the medial head of the gastrocnemius.
| Ligament/Tendons | Anatomic Attachment |
|---|---|
| FCL |
Femoral: 1.4 mm proximal and 3.1 mm posterior to lateral epicondyle Fibular: 8.2 mm posterior to anterior margin of the fibular head |
| PLT | Femoral: Tendon attaches 18.5 mm anterior to FCL in anterior 1/5 of popliteal sulcus |
| BF tendon | Tendon has direct attachments of long and short heads on fibular head. Common BF tendon attaches to posterolateral aspect of fibular styloid |
| ACL |
Femoral:8.5 mm anterior to posterior cartilage margin, 1.7 mm proximal to the bifurcate ridge, 14.7 mm proximal to the distal cartilage margin, 6.1 mm posteriorto the lateral intercondylar ridge Tibial: 7.5 mm medial to the anterior horn of the lateral meniscus |
| PCL |
Femoral: ALB: Between trochlear arch and medial arch point along cartilage margin PMB: 8–9 mm posterior to articular cartilage Tibial: 6–7 mm above champagne glass drop-off at bundle ridge. Avoid shiny white fibers of posterior medial meniscus root. Aligned with medial border of lateral tibial eminence |
| sMCL |
Femoral: 3.2 mm proximal and 4.8 mm posterior to medial epicondyle Tibia: Proximal: 12 mm distal to joint line Distal: 6 cm distal to the joint, anterior to posteromedial tibial crest |
| POL |
Femur: 1.4 mm distal and 2.9 mm anterior to medial GT. 7.7 mm distal and 6.4 mm posterior to adductor tubercle Tibia: Distal to anterior arm of SM tendon, at posterior border of sMCL. |
FCL: Fibular collateral ligament, PLT: Popliteus tendon, BF: Biceps femoris, ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, sMCL: Superficial medial collateral ligament, POL: Posterior oblique ligament
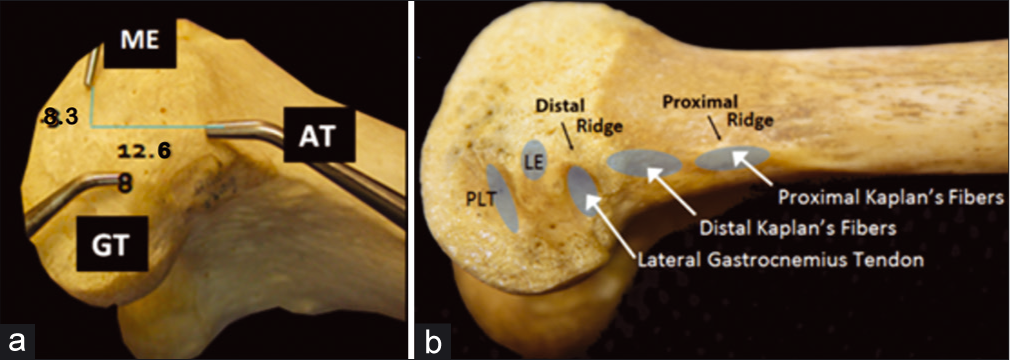
- The distal femur contains a number of bony landmarks visible on the medial (a) and lateral (b) aspects of the distal femur. The adductor tubercle, on which the adductor magnus tendon inserts, is 12.6 mm proximal and 8.3 mm posterior to the medial epicondyle. The gastrocnemius tubercle is 9.4 mm distal and 8.7 mm posterior to the adductor tubercle and 13.7 mm posterior to the medial epicondyle. The medial head of the gastrocnemius originates at this gastrocnemius tubercle just superior to the joint capsule on the lateral medial condyle. ME: Median eminence, AT: Adductor tubercle, GT: Gastrocnemius tubercle,LE: Lateral epicondyle, PLT: Popliteus tendon (sulcus).
The proximal and distal tibial attachments of the sMCL are 12 mm and 6 cm distal to the joint line, respectively.
The distal tibial sMCL attachment is located within the pes anserine bursa, at the posterior aspect of the distal sMCL attachment site.[86] The POL tibial attachment is found just anterior to the direct arm of the semimembranosus tendon. On the distal lateral aspect of the femur, the most relevant landmarks are the PLT and proximal FCL attachments. The PLT attaches in the anterior fifth of the popliteal sulcus, 18.5 mm anterior and distal to the proximal FCL attachment[87] [Figure 6b]. The FCL attachment itself can be found 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle (LE)[8] [Figure 7]. The distal attachment of the FCL can be found within the BF bursa and midway along the lateral fibular head.[5,88]
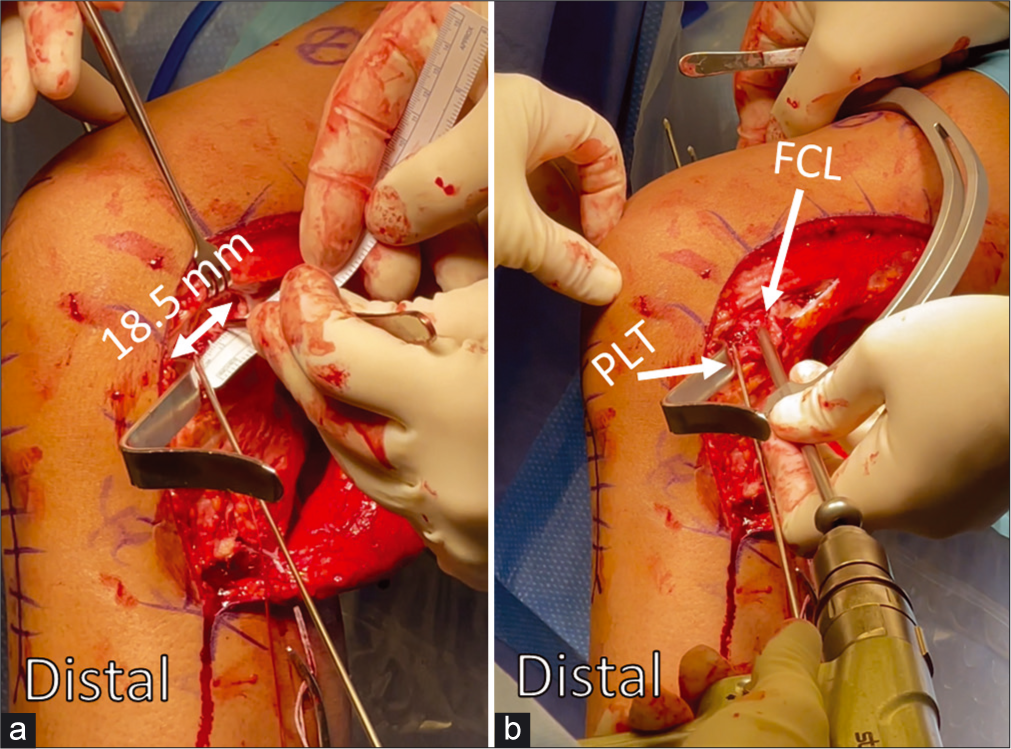
- Anatomic reconstruction of the popliteus tendon (PLT) and fibular collateral ligament (FCL) attachments to the femur is crucial for restoring posterolateral corner integrity during multiple ligament reconstructions. The PLT attaches in the anterior 1/5 of the popliteal sulcus, (a) and a ruler is used to measure 18.5 mm posteriorly to place the proximal FCL tunnel. (b)
In the lateral intercondylar notch, the overall anatomic center of the ACL femoral attachment is 7.1 mm posterior to the LIR and 4.8 mm proximal to the bifurcate ridge[89] [Figure 8]. In the medial intercondylar notch, the center of the PCL ALB is 7.4 mm from the trochlear point and 11.0 mm from the medial arch point and along the edge of the articular cartilage [Figure 9a]. The PMB center is 11.1 mm from the medial arch point, and 10.8 mm from the posterior point.[90] Both bundles of the PCL insert together on the tibia in the PCL Facet, a shallow bony ledge posterior and distal to the tibial plateau, terminating in the champagne-glass drop-off.[89] During PCL reconstruction, the drill guide should be aimed to emerge 1.3 mm proximal to the bundle ridge[89] [Figure 9b and Figure 10].
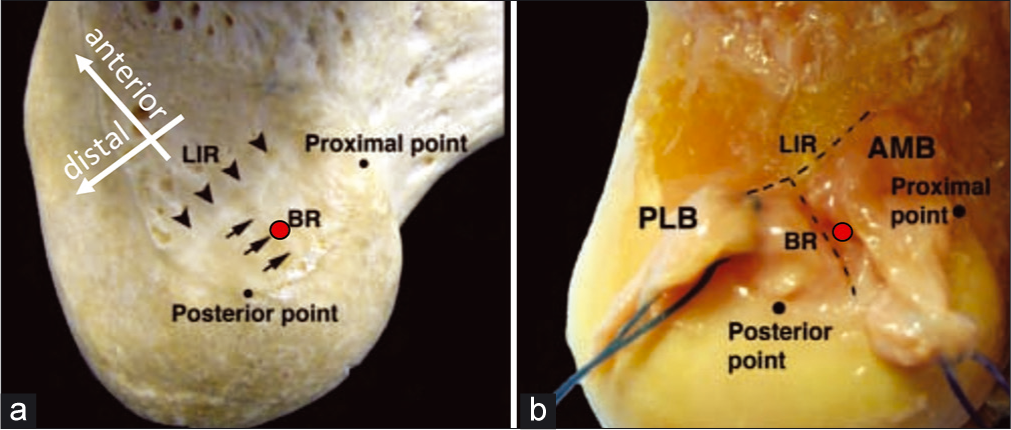
- The Lateral Intercondylar Notch contains the attachments of the anteromedial and posterolateral bundles of the anterior cruciate ligament. The lateral intercondylar ridge (LIR), also known as residents’ ridge, serves as the anterior-most extent of the ACL. (a) The bifurcate ridge (BR) travels from the posterior cartilage margin to the mid-point of the LIR, and separates the ACL bundles. The overall anatomic center of the ACL is indicated with a red circle above; this is the ideal location for the femoral ACL reconstruction tunnel. It is slightly proximal to the BR, and should be as posterior as possible while allowing for a 2 mm backwall after reaming the tunnel with a 10-mm reamer. (b) This image initially appeared in Ziegler CG, Pietrini SD, Westerhaus BD, Anderson CJ, Widjicks CA, Johansen S, Engebretsen L, LaPrade RF. Arthroscopically Pertinent Landmarks for Tunnel Positioning in Single-Bundle and Double-Bundle Anterior Cruciate Ligament Reconstructions. Am J Sport Med. 2011;39(4):743-751.
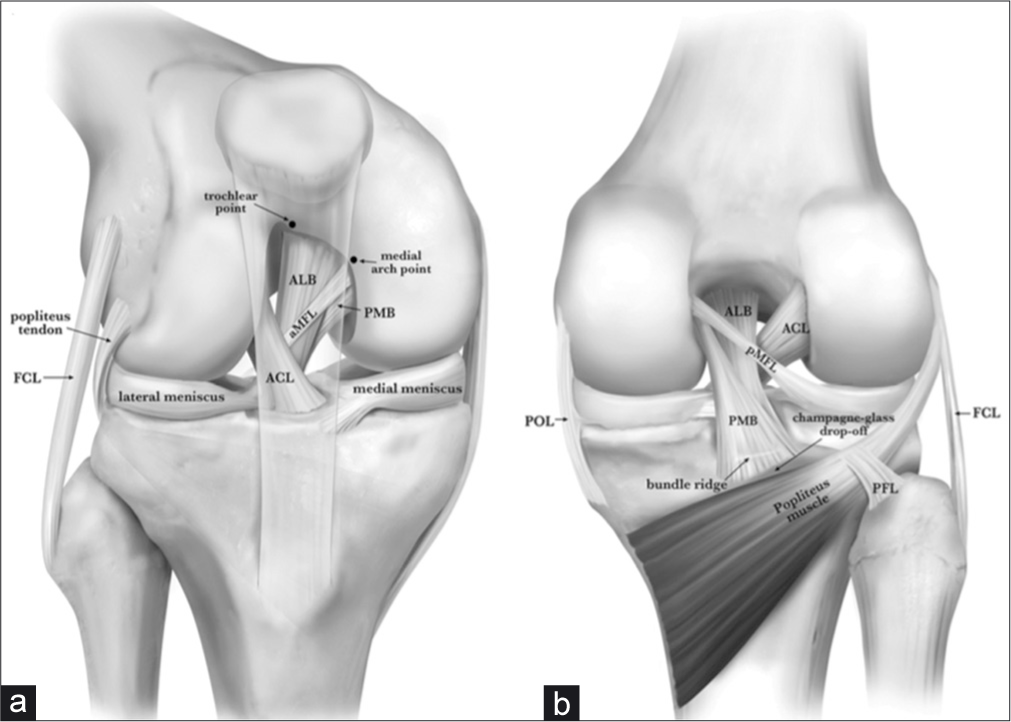
- Anterior (a) and posterior (b) anatomy of the posterior cruciate ligament (PCL). Pertinent anatomy for double-bundle PCL reconstruction (db-PLCR) includes the trochlear point and medial arch point anteriorly (a). The trochlear point marks the proximal extent of the anterolateral bundle (ALB) and the medial arch point represents the distal extent of the ALB along the anterior cartilage margin. Posteriorly, the bundle ridge separates the bundles of the PCL on the PCL facet, just above a bony shelf called the champagne-glass drop-off. Reproduced with permission from Kennedy NI, Wijdicks CA, Goldsmith MT, et al. Kinematic analysis of the posterior cruciate ligament, part 1: the individual and collective function of the anterolateral and posteromedial bundles. Am J Sports Med. 2013;41(12):2828- 2838.
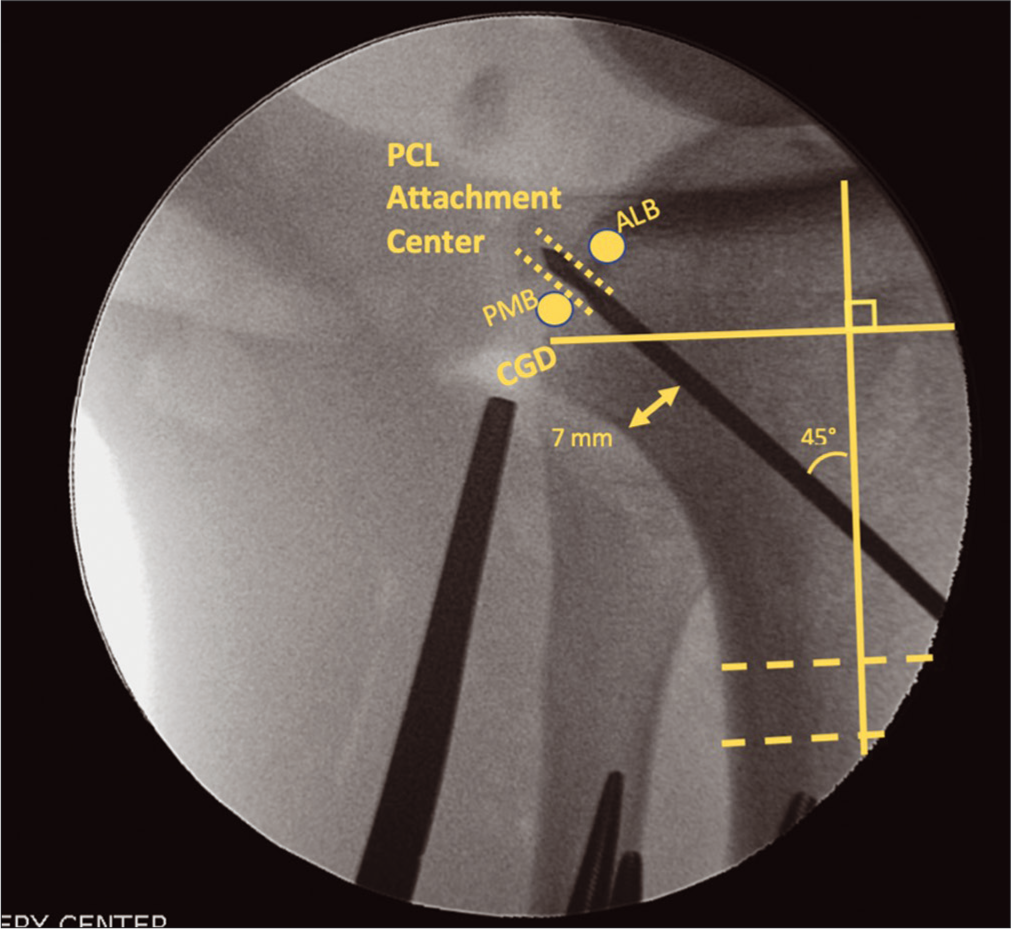
- Aiming the tibial posterior cruciate ligament (PCL) tunnel to intersect with the anatomic PCL attachment center on the PCL facet requires the use of intraoperative fluoroscopy. While two grafts are used to reconstruct the PCL (ALB and AMB), only one tibial tunnel is used due to the close proximity attachment sites of the two bundles on the PCL facet. The guide pin is aimed from the anterior tibia, approximately midway between the anterior tibial crest and the medial border of the tibia. An adequate bone bridge must be maintained posteriorly (minimum 7 mm), and the pin is angles at approximately 45° relative to the long axis of the tibia (as determined above with a two line method). The pin should emerge midway between the ALB and PMB footprints, just above the champagne-glass drop-off. This figure originally appeared in Floyd ER, Carlson GB, Monson JK, and LaPrade RF. Multiple Ligament Reconstructions of the Knee and Posterolateral Corner. Arthroscopy Techniques. 2021;10(5):E1269-E1280.
TUNNEL ORIENTATION
One of the chief concerns in MLKI reconstruction is the sheer number of tunnels traversing the distal femur and proximal tibia [Figure 11]. To decide how to avoid tunnel convergence, Moatshe et al. created CT-based three-dimensional models of knees to determine appropriate trajectories for drilling reconstruction tunnels.[91,92] On the lateral side, FCL tunnels were determined to be most at-risk of convergence with the ACL femoral tunnel. To avoid convergence with one another, the FCL and PLT tunnels are made parallel to one another. To avoid the ACL femoral tunnel, the FCL and PLT femoral tunnels are aimed 35° anteriorly (in the axial plane) with a flat (0°) coronal trajectory.[8,91,93] On the medial side of the distal femur, the sMCL, POL, and double-bundle PCL tunnels have a high risk of convergence. Moatshe et al. recommended aiming the sMCL tunnel 40° anteriorly in the axial plane and proximally in the coronal plane to avoid the ALB of the PCL.[91] The POL tunnel trajectory should be 20° anterior in the axial and proximal in the coronal planes.[8] On the tibia, POL and PCL tunnels have a high risk of convergence when the POL tunnel is aimed directly at Gerdy’s tubercle. Aiming the tibial POL tunnel 15 mm medial to Gerdy’s tubercle, and the tibial sMCL tunnel 30° distally, resulted in no convergence scenarios in these models.[92]
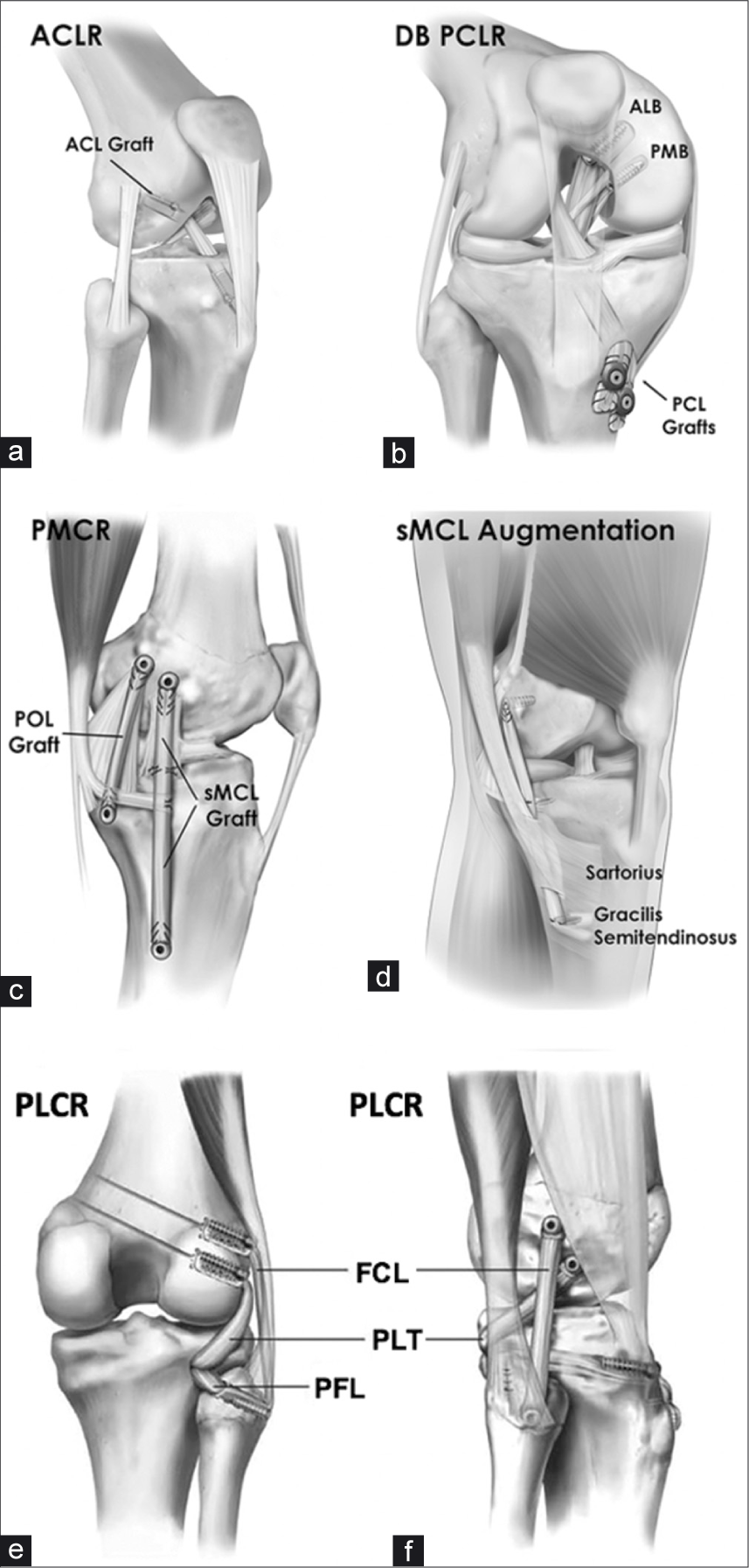
- Reconstruction techniques used for multiligament knee reconstruction. (a) Anterolateral view of a right knee shows a single- bundle ACL reconstruction with a bone-patellar tendon-bone autograft. (b) Double-bundle PCL reconstruction on a right knee with allografts for the anterolateral and posteromedial bundles. The grafts were fixed on the tibia with interference screws and washers. (c) A medial left knee reconstruction, with allografts for sMCL and POL. (d) An sMCL augmentation with gracilis and semitendinosus autograft on a left knee; reconstruction is favored by the authors as it allows for more dependable outcomes. (e) and (f) A full PLC knee reconstruction. ACL: Anterior cruciate ligament, ACLR: Anterior cruciate ligament reconstruction, ALB: anterolateral bundle, DB: double bundle, FCL: Fibular collateral ligament, FCLR: Fibular collateral ligament reconstruction, PCL: Posterior cruciate ligament, PCLR: Posterior cruciate ligament reconstruction, PFL: Popliteofibular ligament, PLCR: Posterolateral corner reconstruction, PMB: Posteromedial bundle, PMCR: Posteromedial corner reconstruction, POL: Posterior oblique ligament, sMCL: Superficial medial collateral ligament, PLC: Posterolateral corner.
GRAFT PASSAGE AND TENSIONING SEQUENCE
Rationale
Not only are the timing, tunnel placement and orientation, and type of graft important, but Moatshe et al. in 2018 reported the results of a biomechanical study detailing the significance of the tibiofemoral orientation and sequence used to tension each individual graft.[94] There are several possible reasons that the orientation and order of securing grafts may be important. For instance, tensioning the PLC grafts first in a multiligament injury results in an abnormal rotational component that cannot be corrected afterwards.[95,96] In addition to providing rationale for double- bundle rather than single-bundle reconstruction, Kennedy et al. (2014) also examined graft tension at a range of flexion angles, concluding that forces across the graft were minimized with the ALB tensioned at 90° and the PMB tensioned in full extension.[79,80] In their analysis of the whole tensioning sequence, Moatshe et al. recommended tensioning the PCL bundles first (ALB first at 90 °and then PMB at 0°), then the ACL, then the PLC (FCL first at 20° and then PFL and PLT at 60°), and finally the MCL and POL. This was based on increased posterior tibial translation observed when the ACL was tensioned first, and greater increases in internal rotation of the tibial observed at all flexion angles when the PLC was tensioned first.[94]
Preparation of the operating room and surgical team involves verifying that the correct instruments and the desired grafts are available. Beginning graft preparation early, ensuring appropriate positioning of the patient to allow for full flexion and extension and performing an examination under anesthesia are preliminary steps to increase operative efficiency.[97]
Overview of graft passage and sequence
In a hypothetical patient with a KD-IV dislocation, the PCL, ALB, and PMB are first secured in their femoral bone tunnels using passing sutures and interference screws. The distal ends of these grafts will be passed down the tibial tunnel [Figure 12]. The ACL graft femoral block is then passed and secured with an interference screw [Figure 13]. The proximal bone blocks for the FCL and PLT grafts are then likewise then secured in their femoral tunnels. The FCL graft is passed under the iliotibial band, then through a fibular head tunnel. The MCL and POL grafts are then passed into their femoral tunnels and secured with 7 × 20 mm interference screws. They are then passed along their anatomical course and into their tibial tunnels. The tibial ends of the PCL grafts are then secured with screws and washers with spikes or large staples. After cycling the knee to remove slack, the ALB graft is secured first at 90° of flexion, followed by the PMB in extension. The ACL graft is tensioned next and secured in the tibia. The FCL graft is then secured in the fibular head (with its tail exiting the posteromedial aperture of the fibular head tunnel) with the knee flexed to 20°, and slight varus and traction forces [Figure 14]. The FCL tail (now serving as PFL graft) and PLT graft are passed posterior to anterior through the tibial PLC tunnel and secured on the anterior tibia [Figure 15]. Finally, the MCL graft is secured in its tibial tunnel with an interference screw while the knee flexed to 20°, the leg in neutral rotation and traction is applied to the graft with gentle varus force. The proximal tibial attachment of the sMCL is reconstituted with a suture anchor placed at the anatomic site, 12.2 mm distal to the joint line, directly medial to the anterior arm of the semimembranosus tendon. The POL is secured with an interference screw in full extension [Figure 16 and Table 4].
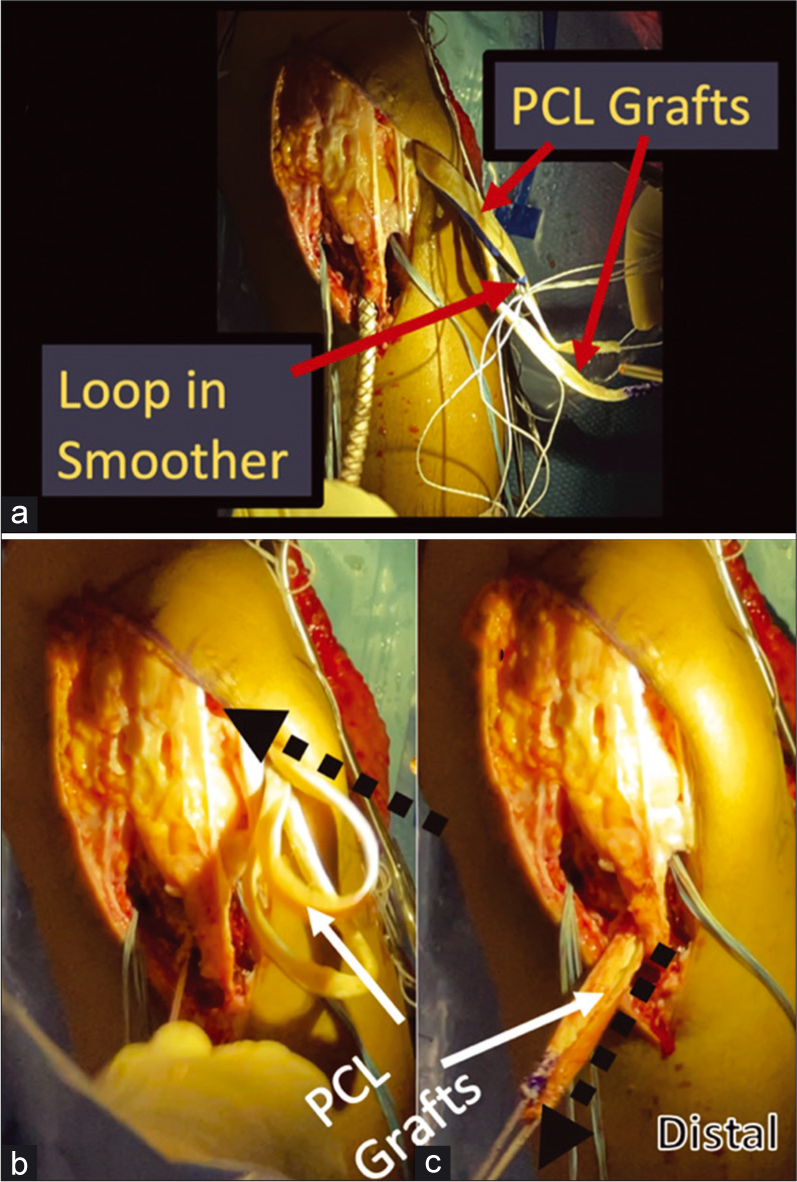
- Passing the PCL grafts using a large smoothing tool. A smoother passed up through the tibial PCL tunnel is used to ease the path of graft passage and brought out through the lateral portal. Once brought out through the lateral portal, the sutures through the tubularized distal ends of the PCL grafts are passed through a loop on the proximal end of the smoother. (Panel a). The smoother is then pulled distally, pulling the distal PCL graft ends back into the joint through the lateral portal. (Panel b) The distal suture ends of the PCL grafts are pulled down the tibial tunnel as the smoother is withdrawn, and then grasped to complete passage of the PCL grafts through the PCL tibial tunnel. (Panel c). PCL: Posterior cruciate ligament.
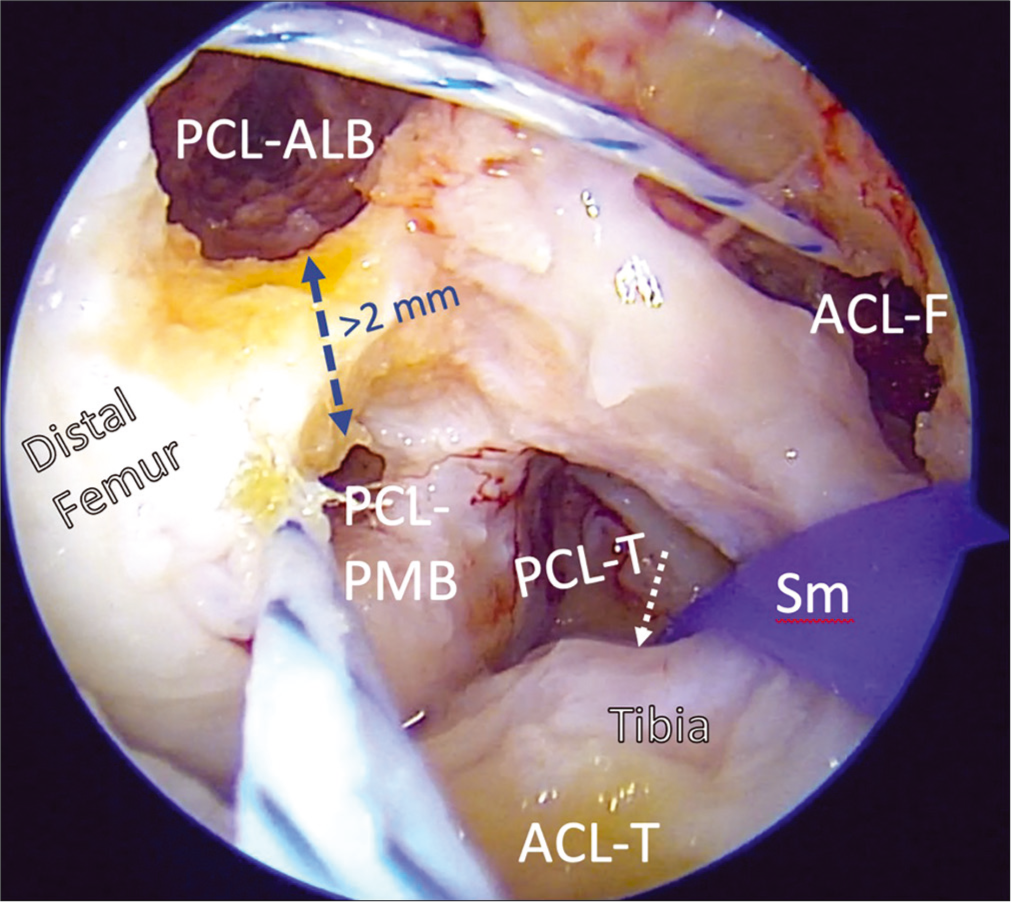
- Creation of PCL and ACL femoral tunnels in the intercondylar notch is challenging, as the number of bony reconstruction tunnels needed in multiple ligament reconstructions may introduce dangers of convergence and blowout. Using arthroscopic landmarks to reconstruct the ACL and PCL anatomically and using validated orientation angles for to drill and overream guide pins is important to prevent these potential complications and restore the native biomechanics of the knee. The PCL grafts (AMB, PMB) are passed and secured in their femoral tunnels first, then will then be passed through the tibial tunnel. Then the ACL graft is passed up through its tibial tunnel and secured in the femur. PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: Posteromedial bundle, ACL-F: ACL femoral tunnel, ACL-T, PCL-T: Tibial tunnels for ACL, PCL, Sm: Large smoothing tool with loop for graft passage.
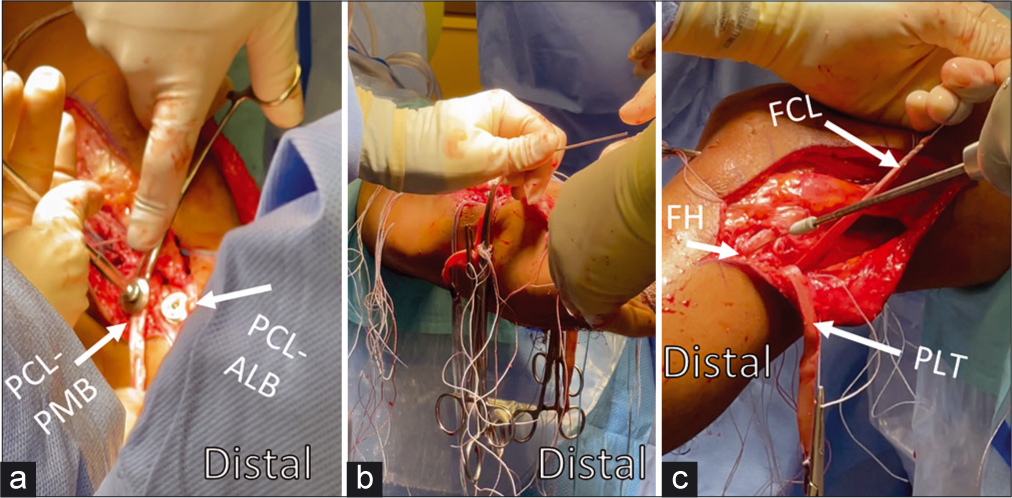
- Graft passage order and positioning for fixation have been established using biomechanical models to approximate native kinematics. In vitro studies have demonstrated the PCL should be tensioned first; tensioning the ACL first leads to abnormal posterior tibial translation, and tensioning the PLC first has been observed to increase tibial internal rotation. After passage, the PCL-ALB graft is secured first at 90° of flexion with neutral rotation and an anterior reduction force to restore step-off. Then the PCL-PMB is secured in extension with a distal traction force applied to the graft (Panel a). The ACL is also secured in extension with distal traction and cycling of the knee to eliminate slack (Panel b). The FCL graft is then secured likewise with distal traction but in 20° of flexion and neutral rotation with a valgus reduction force through the fibular head (Panel c). After both split Achilles allografts (PLC) are passed posterior to anterior through the tibial tunnel, both are secured with the knee in 60° of flexion and with neutral rotation. Assessment of Lachman and range-of-motion can then be assessed to verify appropriate restraint after graft fixation. ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: posteromedial bundle, FCL: Fibular collateral ligament, PLC: Posterolateral corner, FH: Fibular head, PLT: Popliteus tendon
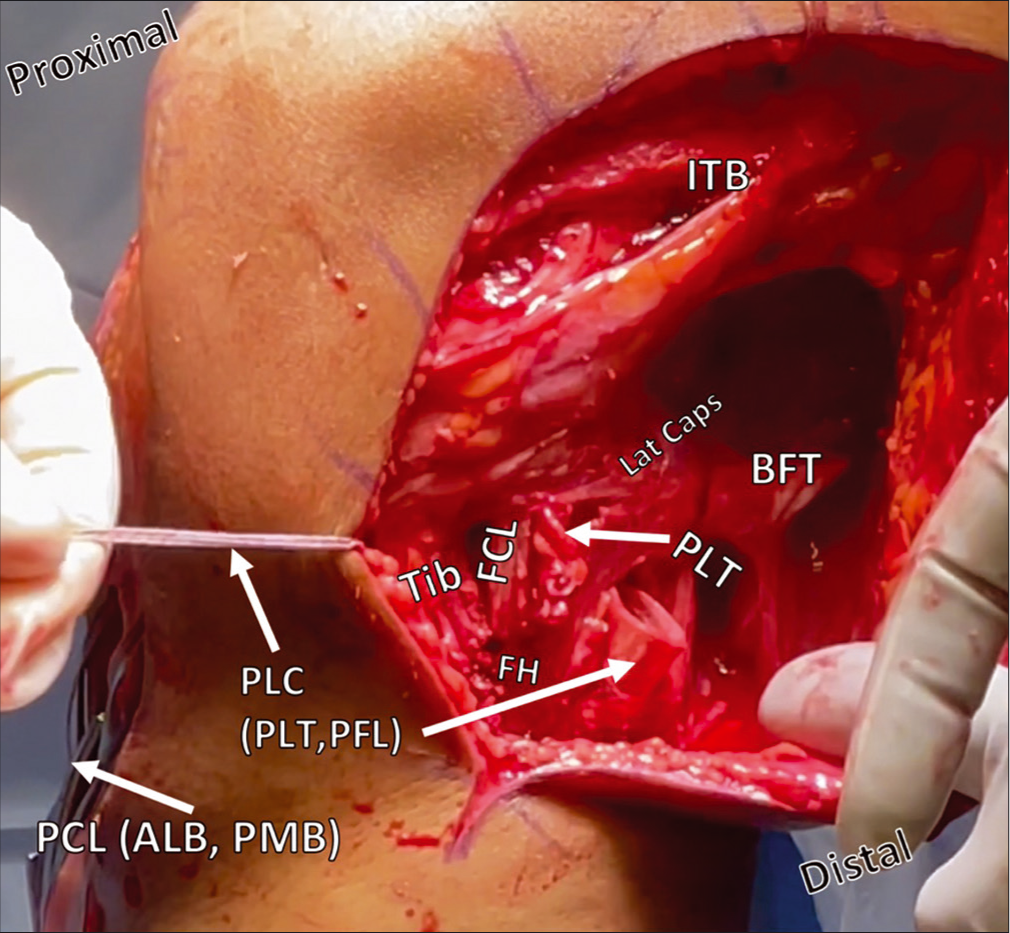
- Posterolateral corner reconstruction during passage of the FCL/PFL and PLT grafts from posterior to anterior through the tibial tunnel. Restoration of the anatomy of the PLC is accomplished with two grafts to reconstruct three ligaments. Above, the FCL has been passed deep to the ITB from its proximal attachment on the femur, and, along with the PLT graft, is being pulled from posterior to anterior through a proximal tibial tunnel. Other structures, including the PCL grafts protruding from the anterior tibial cortex, and the BFT are also labelled. ITB: Iliotibial band, Lat caps: Lateral capsule, BFT: Biceps femoris tendon, PLT: Popliteus tendon, FCL: Fibular collateral ligament, FH: Fibular head, Tib: Tibia, PLC: Posterolateral corner, PCL: Posterior cruciate ligament, ALB: Anterolateral bundle, PMB: Posteromedial bundle.
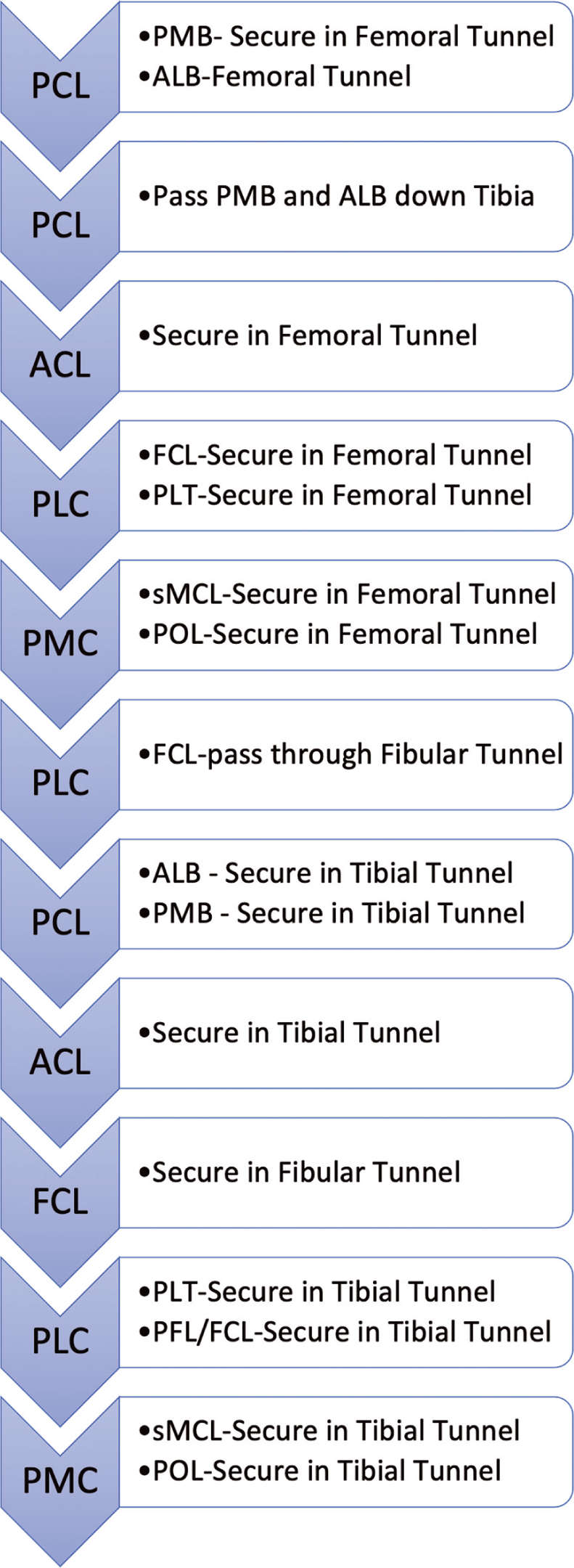
- Order of Graft Fixation When performing reconstruction of extensive injuries around the knee involving both cruciate ligaments and lateral-sided structures, fixation and tensioning of grafts should follow the general order: Posterior Cruciate Ligament (PCL), then Anterior Cruciate Ligament (ACL), then posterolateral corner (PLC) then posteromedial corner (PMC). A more in-depth look at when to secure grafts in femoral tunnels, pass through transosseous tunnels, and secure grafts in their respective tibial fixation points is given in this flow-chart. The heading boxes indicate ACL, PCL, PMC or PLC, and the text boxes indicate what part of those structures and what fixation or graft passages are performed.
| Pearls | Pitfalls |
|---|---|
| Perform open posterolateral corner and medial-sided approaches first to visualize the anatomy before fluid extravasation | Biceps tendon avulsion, posterolateral capsule tears and subsequent scarring can make the common peroneal nerve difficult to locate. Proceed slowly with neurolysis |
| Incise the biceps bursa in order to identify the FCL fibular attachment, 28 mm distal to fibular styloid and 8 mm posterior to anterior border of fibula | Reaming the fibular head tunnel too proximally during PLC reconstruction or failure to maintain 2 mm of backwall during femoral ACL tunnel reaming risks blowout. |
| Tag any remnant FCL to assist in identifying femoral FCL origin | Use a ruler to validate 18.5 mm of distance between femoral PLT and FCL pins prior to reaming, otherwise risk non-anatomic placement or tunnel convergence |
| When drilling the tibial PLC tunnel, place an obturator inside the fibular head tunnel to serve as a guide for the trajectory of the tibia PLC tunnel | If the PLC tibial guide pin is too lateral, it can enter the anterior compartment musculature or the PTFJ, particularly if the patient is obese |
| Tibial PLC tunnel should exit 1 cm medial and 1 cm proximal to posteromedial exit to fibular tunnel | Make the femoral ITB incision anteriorly to prevent soft tissue from being difficult to retract during femoral tunnel prep |
| Use a large Chandler retractor with a finger behind it to protect the neurovascular bundle when drilling PLC tibial tunnel | Ensure PLT and FCL grafts are passed under the ITB; with the PLTis deep to the FCL graft |
| If having difficulty finding FCL femoral origin, make a small arthrotomy and identify PLT femoral origin, then measure 18.5 mm posterior | Aim femoral tunnels 35–40° proximal and posterior to avoid tunnel convergence with an ACL femoral tunnel |
| If needed, repair BF Tendon in full extension after PLC Reconstruction to avoid convergence between suture anchors and fibular tunnel | Remove reamers by hand to avoid damage to neurovascular structures |
| The distal MCL reconstruction graft should be secured at the posterior border of the native sMCL | MCL reconstruction grafts secured to anterior border of sMCL are more likely to fail |
| Incise the pes anserine bursa to find the distal MCL tibial attachment | Fixation screws may damage the distal end of the MCL graft; notch the distal end of the graft before securing with screw |
| Follow the adductor magnus tendon along the distal VMO to the adductor tubercle to locate the MCL and POL attachment sites | Locate the femoral attachments of BOTH the sMCL and the POL before reaming tunnels; otherwise risk nonanatomic tunnel sites |
| Verify correct PCL tibial guide pin position fluoroscopically prior to reaming tunnel | Maintain a 2 mm bone bridge between PCL ALB and PLB socketsor risk blowout |
| Bring distal ends of PCL ALB and PMB grafts out of the joint and loop passing sutures through the proximal end of the smoothing tool to pass through tibia | To avoid over-penetration, ream ~last 40% of tibial PCL and ACL tunnels by hand |
| Ensure 2 mm backwall of ACL femoral tunnel to avoid blowout | Graft-Tunnel mismatch possible if the patellar tendon autograft is unusually long; drop hand to distalize ACL tibial tunnel to accommodate |
ACL: Anterior cruciate ligament, PCL: Posterior cruciate ligament, MCL: Medial collateral ligament, FCL: Fibular collateral ligament, PLT: Popliteus tendon, ALB: Anterolateral bundle, PLB: Posterolateral bundle, sMCL: Superficial medial collateral ligament, VMO: Vastus medialis obliquus, POL: Posterior oblique ligament, ITB: Iliotibial band, PTJF: Proximal tibiofibular joint
REHABILITATION
Variable combinations of injuries, pathologies of bone, soft tissue, ligaments, nerves, and vascular structures make care protocols for these injuries difficult to standardize. An advantage of early, single-stage surgery is the ability to begin early ROM, which helps prevent arthrofibrosis while also maximizing joint health and nutrition. As was previously mentioned, a systematic review by Richter et al. pointed to functional rehabilitation as the most significant prognostic factor in their study.[40]
On post-operative day 1, baseline AP and lateral radiographs are taken and physical therapy begins immediately [Figure 17]. Liberal use of cryotherapy is encouraged to manage pain and swelling. The early stages of therapy include patient education, patellar mobilizations, ROM exercises, and quadriceps activation. Prone, passive ROM is initiated first in patients with PCL injuries to minimize stress on the grafts caused by posterior tibial translation, with a limit of 90° in the first 2 weeks. ROM is then progressed beyond 90° and patients may perform exercises outside of the prone position. However, strategies to minimize posterior tibial translation should continue.

- Multiple Ligament Reconstructions of the cruciate ligaments, posterolateral corner (PLC) and posteromedial corner (PMC). This anteroposterior (AP) radiographic view of a left knee demonstrates anatomic reconstructions of all major stabilizing ligaments of the knee. Titanium screws are used to secure the anterolateral bundle posterior cruciate ligament (PCL) graft, the anterior cruciate ligament graft in the femur and tibia, and the femoral fibular collateral ligament and popliteus tendon grafts in the femur. The distal ends of both PCL grafts are secured with two large staples. The PLC grafts are secured in the fibular head and tibia with radiotransparent bioabsorbable screws, as are the femoral and tibial ends of the superficial medial collateral ligament (sMCL) graft and the posterior oblique ligament (POL).
Patients remain non-weight bearing postoperatively for 6-weeks, and 9–12 months of rehabilitation are generally required before a return to previous levels of activity. Patients transition from an immobilizer immediately after surgery to a brace appropriate for their injury. Patients with ACL but no PCL involvement utilize a static, functional brace (CTi Brace, Ossur, Reykjavik, Iceland). With PCL involvement, patients should be transitioned from the immobilizer to a dynamic PCL brace (Rebound brace, Ossur) at post-operative days 3–5.[98]
In the early post-operative period, repetitive quadriceps muscle contractions induce proximal patellar translation, which, in addition to patellar mobilization, helps minimize potential stiffness in the infrapatellar region. Quadriceps training after surgery first emphasizes muscular activation and then progresses in intensity and structure to recover muscle strength. It should be noted that quadriceps muscle activation at deeper angles of knee flexion (>60°) induces slight posterior tibial translation and closer to extension (<30°) induces more significant anterior translation; these biomechanical properties should be considered with exercise choices per the structures involved in the reconstruction.[99-101] Hamstring muscle activation is restricted in the first 8 weeks to minimize posterior tibial translation and allow for initial tendon healing when a BF tendon repair is required.[100,101]
Isometric muscle training can begin at 8 weeks, initially in shallow angles of knee flexion, and may progress in intensity gradually. Neuromuscular electrical stimulation and blood flow restriction therapy are useful adjuncts to exercise to improve muscle activation and strengthening, reduce muscular atrophy, and possibly mitigate bone density loss following surgery.[102-106] As recovery progresses, the therapy program should evolve in both structure and intensity to continue to promote gains in strength, stability, coordination, and cardiovascular fitness, as tolerated by knee symptoms. Serial physical performance testing, conducted on 3–4 month intervals, provides objective criteria to guide care progression and decision making for return to activity and/or sport.
OUTCOMES AND DISCUSSION
Until recently, data for MLKIs were limited by small cohort sizes, a wide array of treatment algorithms and reconstruction techniques, and a lack of awareness of the importance of immediate postoperative initiation of rehabilitation. A recent study by LaPrade et al. (2019) examined outcomes in 194 patients with low-velocity MLKIs related to athletic activity at an average of 3.5 years follow-up.[1] Reconstruction was with anatomic techniques biomechanically validated to restore native knee kinematics[68,72,81,84] in a single stage, with post-operative day 1 commencement of functional rehabilitation. The authors reported an increase in Tegner activity level from a mean of 1 preoperatively to 6 postoperatively. Western Ontario McMaster Universities Osteoarthritis Index scores decreased on overage from 44 to 3, and Lysholm scores increased from 41 to 90. Outcomes were equivalent no matter which combinations of ligaments were involved, and outcomes were statistically equivalent for acute and chronic repairs.[1] The authors used stress radiography to validate that the early rehabilitation program they adopted did not result in graft attenuation or failure. These results are consistent with the previously described systematic review by Sheth et al. and meta-analysis by Hohmann et al.[12,13] Much of the data described in individual cohorts for MLKIs is from treatment of traumatic knee dislocations, and good outcomes have been reported at follow-up for treatment of these cases as well. Engebretsen et al. (2009) reported on a cohort of 89 patients with knee dislocations at a minimum 2 year follow up.[14] Those authors found a median Lysholm score of 83 and Tegner activity score of 5, although most patients were found to have Kellgren-Lawrence arthritis of grade II or higher at follow-up.
Operative management in the acute phase (<3 weeks) is preferred by the authors, as it decreases scarring present at the time of surgery and allows for faster initiation of rehabilitation, and ultimately faster recovery with less stiffness. Reconstruction techniques for the MCL, POL, PCL, ACL, and PLC have been described with excellent outcomes,[107] but multiligament injuries also come with many associated injuries that need to be addressed; capsular tears, BF, semimembranosus and gastrocnemius tendon avulsions and tears, meniscal and chondral damage, peri- articular fractures, and neurovascular complications all combine to make these tremendously difficult injuries to treat. Having a skilled and knowledgeable team in place, a suitable rehabilitation protocol drawn up, and a thorough operative plan based on the latest research and available data are necessary to make interventions in these cases successful.
CONCLUSION
The existing evidence described above establishes the rationale for early, single-stage operative management of multiple ligament injuries of the knee using anatomically- based reconstruction techniques with immediate commencement of early ROM in the context of a functional rehabilitation program.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
Robert LaPrade: Current Disclosures: Consultant for Arthrex, Ossur, Smith and Nephew and Linvatec Royalties: Arthrex, Ossur and Smith and Nephew Research grants; Smith and Nephew and Ossur Editorial Boards: AOSSM, JEO and KSSTA Committees: AOSSM, AANA, International Society of Arthroscopy, Knee Surgery and Sports Medicine (ISAKOS).
References
- Single-stage multiple-ligament knee reconstructions for sports-related injuries: Outcomes in 194 patients. Am J Sports Med. 2019;47:2563-71.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of knee dislocations. Oper Tech Sports Med. 2003;11:193-8.
- [CrossRef] [Google Scholar]
- High-velocity knee dislocation with vascular injury. Treatment principles. Clin Sports Med. 2000;19:457-77.
- [CrossRef] [Google Scholar]
- Ultra-low-velocity knee dislocations. Am J Sports Med. 2011;39:2170-4.
- [CrossRef] [PubMed] [Google Scholar]
- Multiligament reconstruction of the knee in the setting of knee dislocation with a medial-sided injury. Arthrosc Tech. 2017;6:e341-50.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the outcomes of posterolateral corner knee injuries, part 1: Surgical treatment of acute injuries. Am J Sports Med. 2016;44:1336-42.
- [CrossRef] [PubMed] [Google Scholar]
- Fibular collateral ligament: Varus stress radiographic analysis using 3 different clinical techniques. Orthop J Sports Med. 2018;6:2325967118770170.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of multiligament knee injury: State of the art. J ISAKOS. 2017;2:152-61.
- [CrossRef] [Google Scholar]
- Medial-sided injuries in the multiple ligament knee injury. J Knee Surg. 2020;33:431-9.
- [CrossRef] [PubMed] [Google Scholar]
- An analysis of an anatomical posterolateral knee reconstruction: An in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32:1405-14.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular injuries in knee dislocations: The role of physical examination in determining the need for arteriography. J Bone Joint Surg Am. 2004;86:910-5.
- [CrossRef] [PubMed] [Google Scholar]
- Early or delayed reconstruction in multi-ligament knee injuries: A systematic review and meta-analysis. Knee. 2017;24:909-16.
- [CrossRef] [PubMed] [Google Scholar]
- Early surgery of multiligament knee injuries may yield better results than delayed surgery: A systematic review. J ISAKOS. 2019;4:26-32.
- [CrossRef] [Google Scholar]
- Outcome after knee dislocations: A 2-9 years follow-up of 85 consecutive patients. Knee Surg Sports Traumatol Arthrosc. 2009;17:1013-26.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11:526-9.
- [CrossRef] [Google Scholar]
- A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23:1341-7.
- [CrossRef] [PubMed] [Google Scholar]
- Novel approach for reconstruction of the posterolateral corner using a free tendon graft technique. Sports Med Arthrosc Rev. 2006;14:28-36.
- [CrossRef] [PubMed] [Google Scholar]
- Repair versus reconstruction in acute posterolateral instability of the knee. Sports Med Arthrosc Rev. 2015;23:22-6.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral corner of the knee: Current concepts. Arch Bone Jt Surg. 2016;4:97-103.
- [Google Scholar]
- Incidence of anterior cruciate ligament tears and reconstruction: A 21-year population-based study. Am J Sports Med. 2016;44:1502-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic anterior cruciate ligament reconstruction: A changing paradigm. Knee Surg Sports Traumatol Arthrosc. 2015;23:640-8.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of anterior cruciate ligament injury and other knee ligament injuries: A national population-based study. J Sci Med Sport. 2009;12:622-7.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts of posterolateral corner injuries of the knee. Knee Surg Relat Res. 2017;29:256-68.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the reliability of the dial test for posterolateral rotatory instability: A cadaveric study using an isotonic rotation machine. Arthroscopy. 2008;24:593-8.
- [CrossRef] [PubMed] [Google Scholar]
- Medial knee injury: Part 2, load sharing between the posterior oblique ligament and superficial medial collateral ligament. Am J Sports Med. 2009;37:1771-6.
- [CrossRef] [PubMed] [Google Scholar]
- The management of injuries to the medial side of the knee. J Orthop Sports Phys Ther. 2012;42:221-33.
- [CrossRef] [PubMed] [Google Scholar]
- Injuries to the posterolateral aspect of the knee: Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25:433-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and biomechanics of the medial side of the knee and their surgical implications. Sports Med Arthrosc Rev. 2015;23:63-70.
- [CrossRef] [PubMed] [Google Scholar]
- Medial knee injury: Part 1, static function of the individual components of the main medial knee structures. Am J Sports Med. 2009;37:1762-70.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of valgus stress radiographs with medial knee ligament injuries: An in vitro biomechanical study. Am J Sports Med. 2010;38:330-8.
- [CrossRef] [PubMed] [Google Scholar]
- The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries. An in vitro biomechanical study. J Bone Joint Surg Am. 2008;90:2069-76.
- [CrossRef] [PubMed] [Google Scholar]
- Intraobserver and interobserver reliability of the kneeling technique of stress radiography for the evaluation of posterior knee laxity. Am J Sports Med. 2008;36:1571-6.
- [CrossRef] [PubMed] [Google Scholar]
- Increased accuracy of varus stress radiographs versus magnetic resonance imaging in diagnosing fibular collateral ligament grade III tears. Arthroscopy. 2018;34:2230-5.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral complex knee injuries: Magnetic resonance imaging with surgical correlation. Acta Radiol. 2005;46:297-305.
- [CrossRef] [PubMed] [Google Scholar]
- Location of bone bruises and other osseous injuries associated with acute grade III isolated and combined posterolateral knee injuries. Am J Sports Med. 2010;38:2502-8.
- [CrossRef] [PubMed] [Google Scholar]
- Meniscal root tears: A silent epidemic. Br J Sports Med. 2018;52:872-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mupliple Ligament Knee Injury Treatment Inadequate (Conclusion Statement)
- Decision making in the multiligament-injured knee: An evidence-based systematic review. Arthroscopy. 2009;25:430-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of surgical repair or reconstruction of the cruciate ligaments versus nonsurgical treatment in patients with traumatic knee dislocations. Am J Sports Med. 2002;30:718-27.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of operative and nonoperative treatment of multiligament knee injuries: An evidence-based review. Sports Med Arthrosc Rev. 2011;19:167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Operative versus nonoperative treatment of knee dislocations: A meta-analysis. Am J Knee Surg. 2001;14:33-8.
- [Google Scholar]
- Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17:197-206.
- [CrossRef] [PubMed] [Google Scholar]
- The acutely dislocated knee: Evaluation and management. J Am Acad Orthop Surg. 2004;12:334-46.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple-ligament knee injuries: A systematic review of the timing of operative intervention and postoperative rehabilitation. J Bone Joint Surg Am. 2009;91:2946-57.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of multiple knee ligament injuries in 44 patients: 2-8 years follow-up results. Knee Surg Sports Traumatol Arthrosc. 2006;14:739-49.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of knee dislocations. J Bone Joint Surg Am. 2004;86:262-73.
- [CrossRef] [PubMed] [Google Scholar]
- The posteromedial corner of the knee: An international expert consensus statement on diagnosis, classification, treatment, and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2020;2020:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral corner of the knee: An expert consensus statement on diagnosis, classification, treatment, and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2019;27:2520-9.
- [CrossRef] [PubMed] [Google Scholar]
- Can vascular injury be appropriately assessed with physical examination after knee dislocation? Clin Orthop Relat Res. 2016;474:1453-8.
- [CrossRef] [PubMed] [Google Scholar]
- The value of the ankle-brachial index for diagnosing arterial injury after knee dislocation: A prospective study. J Trauma Acute Care Surg. 2004;56:1261-5.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am. 1977;59:236-9.
- [CrossRef] [Google Scholar]
- Evaluation and treatment principlesof knee dislocations. Oper Tech Sports Med. 2001;9:91-5.
- [CrossRef] [Google Scholar]
- Demographics and injuries associated with knee dislocation: A prospective review of 303 patients. Orthop J Sports Med. 2017;5:2325967117706521.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular and nerve injury after knee dislocation: A systematic review. Clin Orthop Relat Res. 2014;472:2621-9.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of multiligamentous knee injury patterns with associated injuries presenting at a level I trauma center. J Orthop Trauma. 2013;27:226-31.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral corner injuries of the knee: A serious injury commonly missed. J Bone Joint Surg Br. 2011;93:194-7.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical repair of dynamic snapping biceps femoris tendon. Arthrosc Tech. 2018;7:e1129-33.
- [CrossRef] [PubMed] [Google Scholar]
- Meniscal tears and articular cartilage damage in the dislocated knee. Knee Surg Sports Traumatol Arthrosc. 2015;23:3019-25.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management and treatment of the anterior cruciate ligament/posterolateral corner injured knee. Clin Sports Med. 2017;36:105-17.
- [CrossRef] [PubMed] [Google Scholar]
- Role of high tibial osteotomy in chronic injuries of posterior cruciate ligament and posterolateral corner. J Orthop Traumatol. 2011;12:1-17.
- [CrossRef] [PubMed] [Google Scholar]
- Proximal tibial opening wedge osteotomy as the initial treatment for chronic posterolateral corner deficiency in the varus knee: A prospective clinical study. Am J Sports Med. 2007;35:1844-50.
- [CrossRef] [PubMed] [Google Scholar]
- The role of osteotomy for the treatment of PCL injuries. Curr Rev Musculoskelet Med. 2018;11:298-306.
- [CrossRef] [PubMed] [Google Scholar]
- 20-year outcomes of anterior cruciate ligament reconstruction with hamstring tendon autograft: The catastrophic effect of age and posterior tibial slope. Am J Sports Med. 2018;46:531-43.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95-100.
- [CrossRef] [PubMed] [Google Scholar]
- Poor results of anterior cruciate ligament repair in adolescence. Acta Orthop Scand. 1988;59:684-6.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5:e149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging updates on the posterior cruciate ligament: A review of the current literature. Am J Sports Med. 2015;43:3077-92.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: A prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93:1672-83.
- [CrossRef] [PubMed] [Google Scholar]
- Biomechanical analysis of a posterior cruciate ligament reconstruction. Deficiency of the posterolateral structures as a cause of graft failure. Am J Sports Med. 2000;28:32-9.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92:16-22.
- [CrossRef] [PubMed] [Google Scholar]
- Fibular collateral ligament anatomical reconstructions: A prospective outcomes study. Am J Sports Med. 2010;38:2005-11.
- [CrossRef] [PubMed] [Google Scholar]
- A biomechanical study of replacement of the posterior cruciate ligament with a graft. Part II: Forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1997;79:381-6.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the outcomes of posterolateral corner knee injuries, part 2: Surgical treatment of chronic injuries. Am J Sports Med. 2016;44:1616-23.
- [CrossRef] [PubMed] [Google Scholar]
- The posterolateral corner of the knee: Repair versus reconstruction. Am J Sports Med. 2005;33:881-8.
- [CrossRef] [PubMed] [Google Scholar]
- Kinematic analysis of the posterior cruciate ligament, part 1: The individual and collective function of the anterolateral and posteromedial bundles. Am J Sports Med. 2013;41:2828-38.
- [CrossRef] [PubMed] [Google Scholar]
- Kinematic analysis of the posterior cruciate ligament, part 2: A comparison of anatomic single versus double-bundle reconstruction. Am J Sports Med. 2013;41:2839-48.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior cruciate ligament graft fixation angles, part 1: Biomechanical evaluation for anatomic single-bundle reconstruction. Am J Sports Med. 2014;42:2338-45.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior cruciate ligament graft fixation angles, part 2: Biomechanical evaluation for anatomic double-bundle reconstruction. Am J Sports Med. 2014;42:2346-55.
- [CrossRef] [Google Scholar]
- Arthroscopic anatomic single-bundle anterior cruciate ligament reconstruction using bone-patellar tendon-bone autograft: Pearls for an accurate reconstruction. Arthrosc Tech. 2017;6:e1159-67.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of revision with hamstring tendon grafts compared with patellar tendon grafts after anterior cruciate ligament reconstruction: A study of 12,643 patients from the Norwegian Cruciate Ligament Registry, 2004-2012. Am J Sports Med. 2014;42:285-91.
- [CrossRef] [PubMed] [Google Scholar]
- A Hamstring-based anatomic posterolateral knee reconstruction with autografts improves both radiographic instability and functional outcomes. Arthroscopy. 2019;35:1676-85.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical technique: Development of an anatomic medial knee reconstruction. Clin Orthop Relat Res. 2012;470:806-14.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37:1116-22.
- [CrossRef] [PubMed] [Google Scholar]
- The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89:2000-10.
- [CrossRef] [PubMed] [Google Scholar]
- An analysis of an antomical posterolateral knee reconstruction: An in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32:1405-14.
- [CrossRef] [PubMed] [Google Scholar]
- Double-bundle posterior cruciate ligament reconstruction in 100 patients at a mean 3 years' follow-up: Outcomes were comparable to anterior cruciate ligament reconstructions. Am J Sports Med. 2018;46:1809-18.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39:743-52.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94:1936-45.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple ligament reconstruction femoral tunnels: Intertunnel relationships and guidelines to avoid convergence. Am J Sports Med. 2017;45:563-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intertunnel relationships in the tibia during reconstruction of multiple knee ligaments: How to avoid tunnel convergence. Am J Sports Med. 2016;44:2864-9.
- [CrossRef] [PubMed] [Google Scholar]
- How to avoid tunnel convergence in a multiligament injured knee. Ann Joint. 2018;3:93.
- [CrossRef] [Google Scholar]
- The influence of graft tensioning sequence on tibiofemoral orientation during bicruciate and posterolateral corner knee ligament reconstruction: A biomechanical study. Am J Sports Med. 2018;46:1863-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reconstruction of knees with combined cruciate deficiencies: A biomechanical study. J Bone Joint Surg Am. 2003;85:1768-74.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of the integrity of posterolateral structures on tibiofemoral orientation when an anterior cruciate ligament graft is tensioned. Am J Sports Med. 2002;30:796-9.
- [CrossRef] [PubMed] [Google Scholar]
- Preparing the surgical team for a quick and efficient procedure In: LaPrade RF, Chahla J, eds. Evidence-Based Management of Complex Knee Injuries Vol 1. (1st ed). St. Louis, Missouri: Elsevier; 2021. p. :433-48.
- [CrossRef] [Google Scholar]
- Quantification of functional brace forces for posterior cruciate ligament injuries on the knee joint: An in vivo investigation. Knee Surg Sports Traumatol Arthrosc. 2015;23:3070-6.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior cruciate ligament strain and tensile forces for weight-bearing and non-weight-bearing exercises: A guide to exercise selection. J Orthop Sports Phys Ther. 2012;42:208-20.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32:1144-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of tibiofemoral joint forces during open-kinetic-chain and closed kinetic-chain exercises. J Bone Joint Surg Am. 1993;75:732-9.
- [CrossRef] [PubMed] [Google Scholar]
- Blood flow restriction therapy after knee surgery: Indications, safety considerations, and postoperative protocol. Arthrosc Tech. 2018;7:e1037-43.
- [CrossRef] [PubMed] [Google Scholar]
- Quadriceps activation following knee injuries: A systematic review. J Athl Train. 2010;45:87-97.
- [CrossRef] [PubMed] [Google Scholar]
- Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br J Sports Med. 2017;51:1003-11.
- [CrossRef] [PubMed] [Google Scholar]
- Neuromuscular electrical stimulation enhances fracture healing: Results of an animal model. J Orthop Res. 2004;22:382-7.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of neuromuscular electrical stimulation to preserve quadriceps muscle fiber size and contractility after anterior cruciate ligament injuries and reconstruction: A randomized, sham-controlled, blinded trial. Am J Sports Med. 2020;48:2429-37.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple ligament reconstructions of the knee and posterolateral corner. Arthrosc Tech. 2021;10:e1269-80.
- [CrossRef] [PubMed] [Google Scholar]







