Translate this page into:
Orthobiologics for knee osteoarthritis: A review of current practice and future directions
*Corresponding author: Tarkik Thami, Department of Orthopaedics, Postgraduate Institute of Medical Education and Research, Chandigarh, India. thamitarkik@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thami T, Kumar P, Gupta A, Patel S. Orthobiologics for knee osteoarthritis: A review of current practice and future directions. J Arthrosc Surg Sports Med. 2024;5:119-4. doi: 10.25259/JASSM_22_2024
Abstract
Knee osteoarthritis (OA) represents a significant challenge in modern orthopedics due to its prevalence and debilitating impact on patients’ quality of life. Traditional treatment options such as analgesics, physical therapy, and corticosteroid injections have shown varying degrees of efficacy in managing symptoms and controlling disease progression. Of late, there has been a growing interest in orthobiologics as a potential therapeutic approach for knee OA. The existing literature on orthobiologics was searched using the keywords “Orthobiologics,” “Knee,” and “Osteoarthritis”. The term orthobiologics encompasses a wide range of biological substances, including platelet-rich plasma (PRP), Bone Marrow aspiration concentrate (BMAC), Autologous conditioned serum (ACS), mesenchymal stem cells (MSCs), and gene therapeutics which are believed to promote tissue repair and regeneration. This review aims to discuss and compare the existing orthobiologics (for knee OA) and the scope of research to develop better formulations possessing enhanced disease modifying effects in the future. Despite the growing enthusiasm, challenges such as standardization of preparation protocols, optimal dosage, and patient selection criteria remain. Moreover, the long-term effects of orthobiologics on joint structure and function require further investigation.
Keywords
Knee
Orthobiologics
Osteoarthritis
Gene therapy
Platelet-rich plasma
INTRODUCTION
Orthobiologics refer to a group of biologically available compounds usually derived from the human body, which can be used for a variety of musculoskeletal pathologies by virtue of their tissue regenerative and reparative functions.[1] The science of studying orthobiologics is an ever evolving one due to advent of molecular science and biotechnology at such a fast pace. The concept of orthobiologics has gained tremendous popularity over the past three decades leading to a better understanding of their mechanism of action at the cellular level.[1-3] Nonetheless, there is so much yet to be discovered about these bioactive compounds in the coming years. Orthobiologics contain many growth factors and anti-inflammatory cytokines which help in boosting their healing potential, making them amenable for use in orthopedic pathologies such as osteoarthritis (OA); focal cartilage defects/injuries; avascular necrosis of bone; tendinitis; ligament injuries; and plantar fasciitis.[1,3]
Orthobiologics: Why are they necessary?
The osteoarthritic knees have a diminished regenerative potential due to ongoing degenerative changes such as age- related decrease in glycosaminoglycan and proteoglycan levels and poor ability to repair focal cartilage lesions such as softening/fissuring.[2,4] Conservative measures such as activity modification, patient education, and lower limb physiotherapy regimen and injectables such as corticosteroids and hyaluronic acid can aim to tackle early stages of OA [Figure 1]. Nonetheless, these methods can only provide symptomatic relief but can’t alter the natural progression of OA. “Cell based” orthobiologic therapies are then tried as the next line of management as they possess the ability to change joint microenvironment through their chondroprotective and tissue regenerative properties. These orthobiologics merely supplement the body’s inherent healing responses to halt the ongoing pathophysiology of OA. It is rather necessary to boost the natural healing process since the tissue anabolic cascades are weakened in geriatric knees.[3,5]
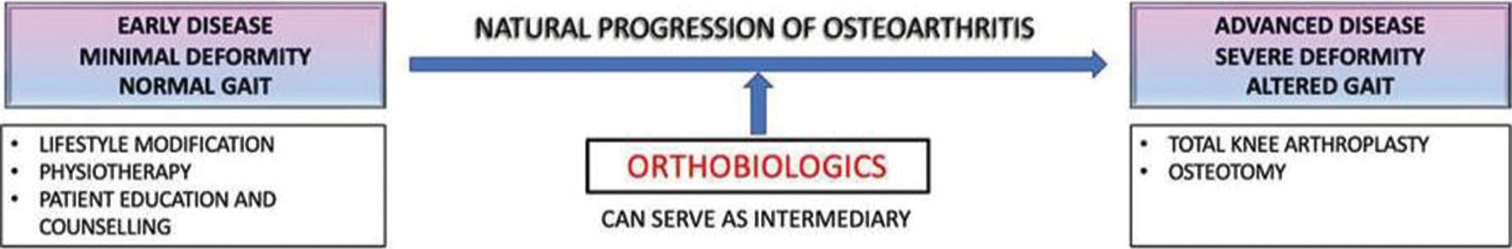
- Role of orthobiologics in preventing the progression of osteoarthritis.
MATERIALS AND METHODS
A comprehensive literature search was performed on June 1, 2024, utilizing databases such as PubMed, Embase, Scopus, and Google Scholar. The search included the keywords “Orthobiologics,” “Knee,” and “Osteoarthritis,” with Boolean operators [AND] and [OR] employed to refine the search. The review focused on full-text articles written in English, excluding duplicates, letters to the editor, case reports, and editorials. The selected articles were then thoroughly evaluated and analyzed for this review.
RESULTS AND DISCUSSION
Classification of orthobiologics (for Knee OA): Despite being a part of routine orthopedic practice since many decades, a comprehensive classification system has not been described in the literature for orthobiologics. A broad range of injectable orthobiologics have been discovered till date.[3,4,6] They can be classified broadly into cellular, acellular, and gene-based therapies [Figure 2]. Some examples of cellular injectable orthobiologics (aka blood derived cellular products) are platelet- rich plasma (PRP); platelet-rich fibrin; bone marrow aspiration concentrate (BMAC); and mesenchymal stem cells (MSCs). Of late, many acellular injectables have also been discovered such as autologous conditioned serum (ACS); stromal vascular fraction (SVF); and alpha-2 macroglobulin (A2M). The gene-based therapies are still under phase-2 and phase-3 trials and have not been marketed for commercial usage.[6]
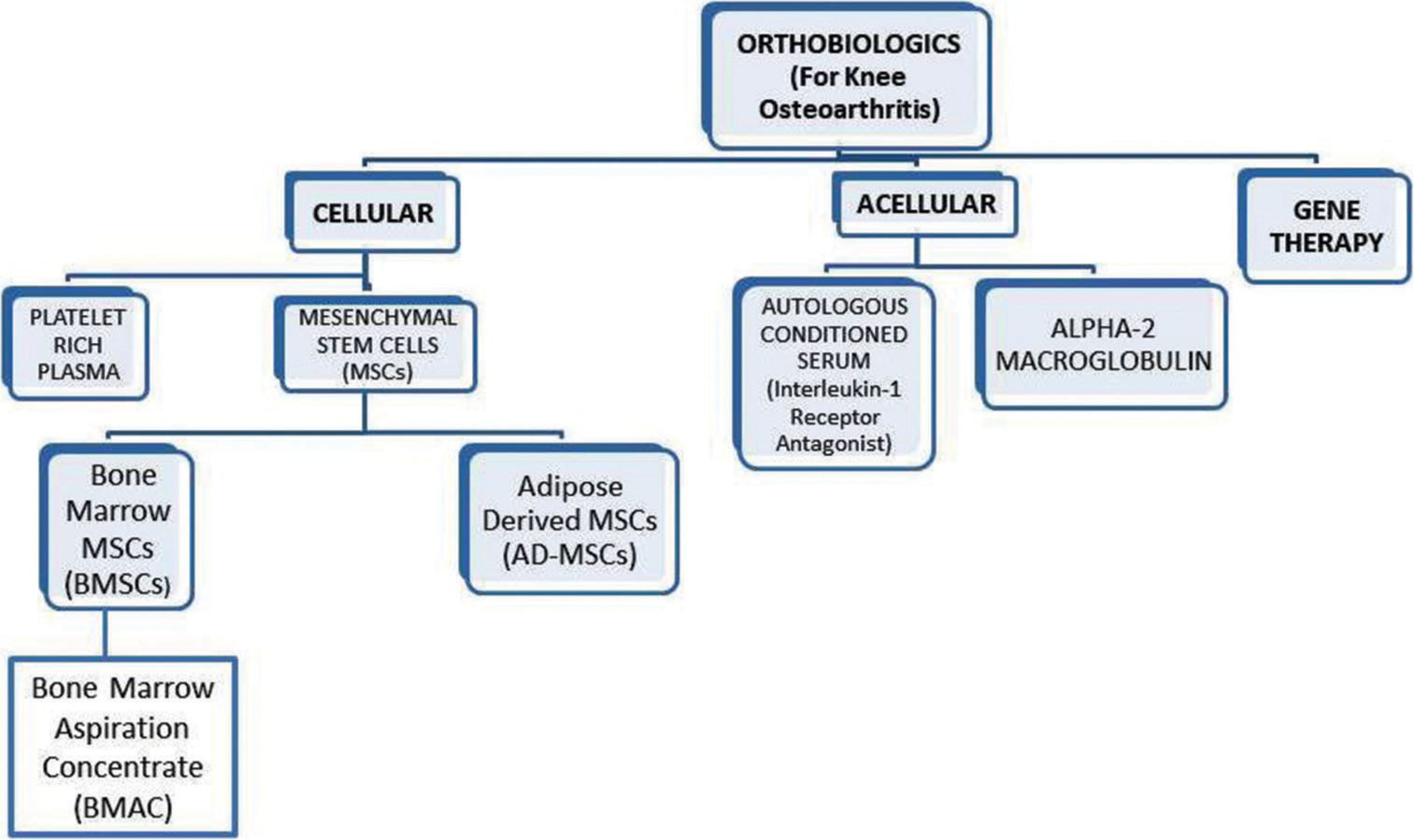
- A simplified classification of injectable orthobiologics for knee osteoarthritis.
PRP
PRP is a regenerative concentrate derived from the patient’s own blood through a process of centrifugation. It effectively contains a high concentration of platelets suspended in plasma making it rich in growth factors which are responsible for its tissue reparative and anabolic action. PRP also holds the potential to counteract catabolic cascades at the cellular levels through its anti-inflammatory cytokines such as transforming growth factor-b (TGF-b), Interleukin (IL)-10, and IL- 13.[7] Over the course of years, PRP has widely emerged as the most commonly used orthobiologic agent for knee OA since it can act directly on cartilage microenvironment by up regulating the anabolic signals and down regulating the catabolic signals which influence cartilage degeneration. PRP is believed to stimulate the body’s natural healing responses, promoting tissue regeneration, counter acting inflammation, and potentially slowing down the progression of degenerative conditions like OA.[7,8] PRP contains a high concentration of many growth factors such as platelet-derived growth factor; insulin-like growth factor; fibroblast growth factor; and TGF-b.[1-3,5] The earliest publications discussing PRP therapy for knee OA began to emerge in the mid-2000s. Patel et al.[5] did one of the initial randomized controlled trials to compare the effects of intra-articular PRP to saline placebo injection. They reported clinically significant improvement in WOMAC scores at sequential follow-up visits and concluded that PRP injections can exhibit short-term effectiveness (up to 6 months for a single dose) in early knee OA. Bansal et al.[7] compared the effects of hyaluronate injection with a PRP injection containing higher platelet count (10 billion) and reported clinically significant results at 1 year follow-up duration.
Even though there is surmounting evidence on the clinical supremacy of PRP injections for knee OA; yet there are numerous variables which remain elusive. Some examples are leukocyte rich versus poor PRP; PRP without or without an activator;[9] low dose versus superdose PRP formulation; single-spin or double-spin centrifugation methods for PRP preparation; and multiple PRP injections versus a single PRP injection. We believe that future studies should focus on evaluation of these variables in an effort to define the ideal PRP formulation that can exist.
The therapeutic use of PRP has many advantages such as the ease of availability of commercial kits; minimal risk of allergic reactions; good patient compliance; and flexibility to choose the required therapeutic dose. Although most of commercially available kits yield 3–5 mL of injectable PRP, recently published literature has also focused on the beneficial effects of a higher volume of PRP (7–8 mL of final product) which contains a relatively higher platelet count (>5 billion absolute platelet numbers).[10] The analogy of “superdose” PRP especially holds true for the knee, since 7–8 mL of injectable drug can diffuse easily inside such a large joint.
PRP preparation techniques
PRP should be prepared with utmost care so as to maintain platelets in their natural resting state. An atraumatic single prick blood sample collection from the ante-cubital vein is preferable. The volume of blood sample collected depends on the dose of PRP you plan to inject. Usually, 25–30 mL of blood is aspirated for a standard 3–5 mL PRP intra-articular injection and 50–60 mL blood is aspirated for a superdose injection (7–8 mL). There are two different methods of centrifugation, namely, single-spin versus double-spin platelet pellet method. Although there is no major difference in outcome parameters between the two centrifugation methods (Filardo et al.),[4] a double-spin method is usually preferred at most centers. It involves subjecting the whole blood to a single spin which lasts for 15 min at 1300 rpm, followed by separation of the supernatant rich in platelets and some white blood cells which are, further, subjected to the second spin. The second spin lasts for 5 min at 2300 rpm resulting in formation of a platelet pellet at the bottom suspended in plasma. The plasma poor plasma is separated as a supernatant and discarded. The ultimate product is now available for intra-articular injections.
BMAC
BMAC has become a crucial biological tool for orthopedic surgeons, being among the few methods approved by the Food and Drug Administration for delivering stem cells and growth factors.[11] BMAC is another “cellular” orthobiologic injectable derived from patient’s own blood through a specialized aspiration needle. BMAC intra-articular injections have shown encouraging results for the treatment of early knee OA and focal cartilage defects.[11,12] BMAC is prepared by collecting marrow aspirate from an easily accessible site such as the anterior superior iliac spine or the posterior superior iliac spine, followed by centrifugation at a high speed (3200 rpm for 15 min) to yield an ultra- filtrate rich in pluripotent MSCs which can differentiate into chondroblastic lineage to hasten and upregulate the cartilage repair cascades inside the knee joint.[11,13] However, in bone marrow aspirates, MSCs make up only a minute fraction (<0.01%) of mononuclear cells after density gradient centrifugation to eliminate red blood cells, granulocytes, immature myeloid precursors, and platelets.[14] By virtue of its high content of growth factors, bioactive proteins, and cartilage precursor cells, BMAC holds enormous potential to alter the course of OA rather than just control pain. However, the existing literature on BMAC is scarce and varies widely in terms of indications, timing, and outcomes.
Kim et al.[12] evaluated the effect of BMAC in 75 knees suffering from OA ranging from Kellgren Lawrence grade I– IV, through visual analog scale (VAS) score; International knee documentation committee (IKDC) score; Knee injury and osteoarthritis outcome score (KOOS); and Lysholm Knee questionnaire. They concluded that BMAC injections led to clinically significant improvement in score values at 6 and 12 months, more so for K.L grades I–III. Shapiro et al.[15] studied the effect of BMAC in a placebo-controlled trial by injecting one knee with BMAC and the other knee with saline placebo injection in 25 patients suffering from bilateral OA. Osteoarthritis research society international (OARSI) and VAS pain scores decreased significantly in both groups at 1 week, 3 months, and 6 months follow-up, but no difference was found between the two groups. Some other studies have reported clinically significant outcome of BMAC for focal cartilage defects when used to augment other treatment options such as microfracture creation.[16]
ACS
IL-1 is a key mediator of pathophysiology of degenerative changes in knee OA and its high levels have been detected in synovial fluid of such knees. The chondrocytes and fibroblasts in an osteoarthritic knee express higher levels of IL-1 receptor type 1. The endogenous inhibitor of IL-1, known as the IL-1 receptor antagonist (IL-1Ra), holds the potential to restrict the intra-articular effects of IL-1 and shield cartilage from matrix metalloproteinase (MMP).[17,18] Numerous researchers have documented the efficacy of IL-1Ra through intra- articular administration in studies involving dogs (Pelletier et al.) affiicted with OA and in initial human trials.[19]
ACS is prepared from patient’s own blood, which has recently gained attention as a potential treatment for knee OA. ACS contains a high level of IL-1Ra. The research on ACS can be dated back to early 2000s. Meijer et al. observed that when blood comes into contact with glass beads, it triggers a robust and swift rise in the production of various anti-inflammatory cytokines, including IL-1Ra.[20] This finding forms the foundation for generating ACS, which is administered into the joint through a course of six intra-articular injections, given twice weekly over a span of 3 weeks. At present, this treatment is accessible for human patients in multiple European nations, and its application is even more prevalent for managing equine OA.[21]
Baltzer et al.[22] conducted a placebo controlled trial on a total of 376 patients, to compare the effects of ACS, hyaluronate, and placebo (saline) as three separate groups. This was the first ever conducted clinical trial on ACS; they evaluated the outcomes based on WOMAC index, VAS score, and SF-8 at 7, 13, and 26 weeks. The outcomes of ACS treatment showed greater efficacy compared to both hyaluronate and saline across all evaluated parameters and time intervals, with improvements reaching clinically significant levels. Notably, there was no discernible difference in efficacy between hyaluronate and saline.
Future advancements in biotechnology can pave the path for improved ACS formulations that optimize growth factor concentrations and biological activity.
A2M
A2M is a broad-spectrum protease inhibitor which primarily works as an anti-inflammatory mediator to prevent the progression of knee OA. A2M is present in both serum as well as synovial fluid, but its intra-articular levels are low in OA. As we have already mentioned, there is a heightened expression of proinflammatory cytokines such as IL-1, IL-6, tumor necrosis factor-alpha, and IL-8 in the synovial fluid of osteoarthritic knees.[23,24] A2M acts as a scavenger protein since it possesses the ability to target proteinase inhibitors such as MMP-13 and adam metallopeptidase with thrombospondin type 1 (ADAMTS-1) to form immune complexes to render these enzymes functionally inactive.[23-26] These immune complexes are subsequently cleared off by synovial cells and macrophages. A2M is prepared by subjecting PRP to a process of ultra-centrifugation to removed platelets and other small molecules, leaving behind plasma which has a high concentration of A2M which is relatively larger molecule.
Wang et al.[26] demonstrated in their study that rats subjected to anterior cruciate ligament transection and treated with intra-articular A2M exhibited reduced MMP-13 levels and a slower progression of OA. Their findings suggest that additional intra-articular A2M could potentially protect chondral tissue in cases of post-traumatic OA.
Klein et al.[27] conducted an RCT to compare intra-articular injections of steroids, PRP, and A2M in 75 patients by evaluating VAS score, WOMAC score, KOOS score, and Lysholm scores. Patient reported outcomes were recorded at 6 weeks and 12 weeks. The A2M group exhibited the highest decrease in VAS score at 6 weeks follow-up. They reported a mildly better effect of steroid and A2M as compared to PRP, but the results were not statistically significant.
Moving forward, personalized therapies utilizing autologous A2M enrichment technology are poised for clinical implementation. This approach allows OA patients to utilize A2M extracted from their own plasma to reduce inflammation levels within their knee joints. Looking ahead, advancements in molecular engineering and biological technology may enable the development of highly effective variants of A2M, akin to the progress seen with synthetic insulin, offering enhanced quality and quantity.
MSCs
Adipose-derived MSCs (AD-MSCSs)
The human body has a huge store of adipose tissue which serves as a prime store of body’s energy reserves. Some recently published literature has tried to explore the other functions of adipose tissue and its contents.[28] It is highly vascular and contains a significant amount of adipose-derived MSCs. These AD-MSCs have recently gained interest due to their paracrine and multipotent ability to differentiate into various cellular lineages.[29] These MSCs usually remain in a dormant stage inside tissues and get activated only when a tissue injury occurs and cytokines are released which ultimately signal these MSCs. By virtue of their paracrine properties, these MSCs get involved in regulating tissue stress signals, down regulation of cellular apoptosis, and enhancement of extra- cellular matrix production. They release anti-inflammatory cytokines to counter-act the inflammation cascade.[29] AD- MSCs are usually derived as a result of lipo-aspiration from high fat storage areas of the body such as abdomen or gluteal region. An aspiration cannula is (inserted in the subcutaneous fat) attached to a vacuum to aspirate the contents. The resultant aspirate is organized into cellular suspensions usually known as SVF or microfragmented adipose tissue (MFAT). [2,29,30] The aspiration cannula has microfilters which process the aspirated fat content, hence signifying the nomenclature of MFAT. The aspirated content is subjected to enzymatic degradation by collagenase to remove unwanted fibrous tissue and other impurities.
Toghraie et al.[31] studies the effects of AD-MSCs in 20 white New Zealand rabbit animal model of knee OA. On radiographic evaluation, they observed decreased osteophyte formation, low cartilage wear, and subchondral sclerosis. Spasovski et al.[32] used a single injection of AD-MSCs in nine patients suffering from knee OA and followed them up till 18 months. They reported significant improvement of all outcome measures at 6 weeks, which continued till the final follow-up.
Therefore, the existing literature suggests that AD-MSCs can be used a promising orthobiologic agent in the future, but there is a need for more robust studies (ideally with a control group) to investigate the long-term benefits of AD-MSCs.
Gene therapy
Gene therapy is an upcoming treatment option for management of knee OA. This technique involves intra-articular delivery of the drug which directly targets the genes involved in the pathogenesis of OA.[33] Their mechanism is of action involves blocking the inflammatory cascade inside the affect joint. Gene therapy can broadly be divided into in vivo and ex vivo treatment arms. The rationale behind gene therapy lies in its potential to address underlying disease mechanisms rather than merely alleviating symptoms. Various delivery systems are being explored to facilitate intra-articular gene transfer, including viral vectors (e.g., lentivirus) and non-viral vectors (e.g., nanoparticles).[34] The in vivo delivery system involves the direct transfer of vector carrying the gene to the target region, often by intra-articular injections. The ex vivo is a rather complicated process since it involves extraction of chondrocytes and other mesenchymal pluripotent cells from the joint. The second step allows their genetic modification outside the body in a sterile environment followed by repeat transfer into the targeted joint by another intra-articular injection. Out of the two delivery systems, “ex vivo” transfer was the first one to be introduced but gradually it was less favored after introduction of the “in vivo” process. The in vivo process allows for increased local gene expression inside the joint, and thus, it can directly act on the pathophysiology of OA at the genomic level.[34] The adeno-associated virus has shown promising results as a vector for in vivo gene transfer.[34,35]
Current research into gene therapy for knee OA is in its early phases, primarily emphasizing safety, feasibility, and initial effectiveness. Addressing challenges such as vector immunogenicity and determining optimal dosing schedules is crucial for ensuring the safety and sustained efficacy of this treatment method. Gene therapy aims to target the fundamental molecular processes involved in the disease, potentially offering treatments that modify its progression. Continued exploration and development are essential to fully realize the therapeutic potential of gene therapy in clinical practice.
Future perspectives
Proteasome-based therapy – An upcoming treatment option
Proteasomal therapy is a newly discovered treatment option for knee OA. The ubiquitin proteasome pathway (UPP) is a group of proteolytic enzymes involved in cellular degradation of articular cartilage proteins. Increased levels of substance–P (SP) and calcitonin gene related peptide (CGRP) are a potential cause of pain seen in osteoarthritic knees.[36] The function of UPP is directly proportional to levels of SP and CGRP in diseased knees. In light of this new understanding, proteasomal inhibition has been touted as topic of research for alleviation of pain in arthritic knees. MG132 is one such potent inhibitor of the proteasome pathway which is being studied as a potential treatment option.[37]
Ahmed et al.[37] studied the effects of MG132 to alleviate joint pain and inflammation in a chemical model of rat OA. Immunohistochemistry (IHC) was used to assess the levels of p50 nuclear factor-kappa B, SP, and CGRP. The group treated with MG132 reported significantly lower IHC staining of all the above-mentioned inflammatory variables.
Further studies may be required to thoroughly investigate the role of MG132 as a potential pharmacological option for treatment of human knee OA.
Limitations
Despite the potential for significant advancements in orthobiologics’ related research, several limitations still persist. Many studies suffer from small sample sizes, which restrict their statistical power and the generalizability of their results. In addition, the lack of standardization in the preparation and administration of orthobiological formulations leads to variability in reported outcomes. Inconsistent reporting of adverse events further complicates the comprehensive assessment of orthobiologics’ safety profile. Moreover, only a few studies have made direct comparisons between different orthobiologic treatments or between orthobiologics and placebos, hindering the determination of their relative efficacy.
To provide more conclusive evidence on the efficacy and safety of orthobiologics in clinical practice, it is crucial to address these limitations through well-designed, large-scale studies that utilize standardized protocols and include longer follow-up periods.
CONCLUSION
The science of orthobiologics is a promising frontier in addressing knee OA, offering advantages beyond temporary symptomatic relief. PRP, MSCs, ACS, and gene therapy are key therapies demonstrating potential in promoting tissue repair and disease modifying effects. While clinical outcomes may vary, these injectables have shown effectiveness in reducing pain and improving joint function, especially in early to moderate arthritis. As the field progresses, integrating orthobiologics into standard management of OA holds promise for improving patient’s quality of life but also to potentially modify the natural course of disease. Continued investigation through clinical trials is crucial to validate these treatment modalities and establish their role in the future treatment of OA.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Orthobiologics in knee osteoarthritis, dream or reality? Arch Orthop Trauma Surg 2024 https://doi.org/10.1007/s00402-024-05310-9
- [CrossRef] [PubMed] [Google Scholar]
- The future of injectable orthobiologic substances for knee osteoarthritis: Options beyond platelet-rich plasma. J Musculoskeletal Surg Res. 2020;4:173.
- [CrossRef] [Google Scholar]
- Current state of platelet-rich plasma and cell-based therapies for the treatment of osteoarthritis and tendon and ligament injuries. J Bone Joint Surg Am. 2022;104:1406-14.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: Single-versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20:2082-91.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-64.
- [CrossRef] [PubMed] [Google Scholar]
- Osteoarthritis gene therapy in 2022. Curr Opin Rheumatol. 2023;35:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci Rep. 2021;11:3971.
- [CrossRef] [Google Scholar]
- Chondroprotective effects of a single PRP injection in a spontaneous osteoarthritis model of Dunkin Hartley Guinea pig: An immunohistochemical analysis. Indian J Orthop. 2024;58:887-93.
- [CrossRef] [PubMed] [Google Scholar]
- Is there a need for an exogenous activator along with PRP for early knee osteoarthritis? A triple-blinded randomized control trial. Indian J Orthop. 2024;58:905-13.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of conventional dose versus superdose platelet-rich plasma for knee osteoarthritis: A prospective, triple-blind, randomized clinical trial. Orthop J Sports Med. 2024;12:23259671241227863. 10.1177%2F23259671241227863 [Last accessed on 2024 Jun 09]
- [CrossRef] [PubMed] [Google Scholar]
- Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: A systematic review of outcomes. Orthop J Sports Med. 2016;4 10.1177/2325967115625481. [Last accessed on 2024 Jun 09]
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24:1505-11.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6:e441-5.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55-62.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2017;45:82-90.
- [CrossRef] [PubMed] [Google Scholar]
- One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: Results at 2-year follow-up. Cartilage. 2011;2:286-99.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring causal correlations between inflammatory cytokines and knee osteoarthritis: A two-sample Mendelian randomization. Front Immunol. 2024;15:1362012.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-1β signaling in osteoarthritis-chondrocytes in focus. Cell Signal. 2019;53:212-223.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012-9.
- [CrossRef] [PubMed] [Google Scholar]
- The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res. 2003;52(10):404-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290-6.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:152-60.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage. 2008;16:1413-20.
- [CrossRef] [PubMed] [Google Scholar]
- Alpha-2-macroglobulin, a native and powerful proteinase inhibitor, prevents cartilage degeneration disease by inhibiting majority of catabolic enzymes and cytokines. Curr Mol Biol Rep. 2021;7:1-7.
- [CrossRef] [Google Scholar]
- Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Arthritis Res Ther. 2017;19:175.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of α2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheumatol. 2014;66:1843-53.
- [CrossRef] [PubMed] [Google Scholar]
- Alpha-2-macroglobulin not significantly better than regular PRP for knee arthritis symptoms. Orthop J Sports Med. 2020;8(7 Suppl 6) 10.1177/2325967120S00454. [Last accessed on 2024 Jun 09]
- [CrossRef] [Google Scholar]
- Intra-articular mesenchymal stem cell injection for knee osteoarthritis: Mechanisms and clinical evidence. Int J Mol Sci. 2022;24:59.
- [CrossRef] [PubMed] [Google Scholar]
- Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13:295-307.
- [CrossRef] [PubMed] [Google Scholar]
- Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg Sports Traumatol Arthrosc. 2019;27:2003-20.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee. 2011;18:71-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med. 2018;20:e3002.
- [CrossRef] [PubMed] [Google Scholar]
- Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294-311.
- [CrossRef] [PubMed] [Google Scholar]
- New treatment for osteoarthritis: Gene therapy. Precis Clin Med. 2023;6:pbad014.
- [CrossRef] [PubMed] [Google Scholar]
- Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14:247-56.
- [CrossRef] [PubMed] [Google Scholar]
- Suppression of pain and joint destruction by inhibition of the proteasome system in experimental osteoarthritis. Pain. 2012;153:18-26.
- [CrossRef] [PubMed] [Google Scholar]
- Attenuation of pain and inflammation in adjuvant-induced arthritis by the proteasome inhibitor MG132. Arthritis Rheum. 2010;62:2160-9.
- [CrossRef] [PubMed] [Google Scholar]






