Translate this page into:
History of hip arthroscopy

*Corresponding author: Vikas Khanduja, Cambridge Hip Preservation Service, Department of Trauma and Orthopaedics, Addenbrooke’s - Cambridge University Hospital, Box 37, Hills Road, Cambridge CB2 0QQ, United Kingdom. vk279@cam.ac.uk
-
Received: ,
Accepted: ,
How to cite this article: Shukla S, Pettit M, Sunil Kumar KH, Khanduja V. History of hip arthroscopy. J Arthrosc Surg Sport Med 2020;1(1):73-80.
Abstract
Hip arthroscopy is a minimally invasive therapeutic and diagnostic procedure appropriate for an evolving list of conditions. It is routinely used for the treatment of intra- and extra-articular pathology of the hip joint. The development of endoscopy paved the way for the development of arthroscopy. Hip arthroscopy was first described in 1931 by Michael Burman, and its widespread adoption was only achieved some 60 years later during the 1990s. Dr. Watanabe, from Japan, has been credited with the development of modern arthroscopy for his work in developing a practical arthroscope and advancement of both explorative arthroscopy and surgical arthroscopic techniques. More recently, the use of distraction proved as a significant step in the utility of hip arthroscopy and paved the way for future innovations in the procedure. The authors provide a brief overview of the history hip arthroscopy, relevant developments which have paved the way for this procedure and the current state of arthroscopy as a diagnostic and therapeutic procedure.
Keywords
Arthroscopy
Hip
History
INTRODUCTION
Hip arthroscopy is a minimally invasive therapeutic and diagnostic procedure which is routinely used for the treatment of intra- and extra-articular pathology of the hip including femoroacetabular impingement (FAI), labral and chondral pathology, ligamentum teres injuries, synovial chondromatosis, internal snapping hip, sub-spinous impingement, gluteus medius tears, and the indications are ever expanding.[1,2] Its popularity has risen dramatically over the past two decades since the association between FAI and osteoarthritis was first described by Ganz et al.[3] Between 2002 and 2013, there was more than a 7-fold increase in the number of arthroscopic hip procedures being performed in the UK.[1] This trend is mirrored in the United States, where femoroplasty, labral repair, and acetabuloplasty are the three most common hip arthroscopic procedures performed.[4] This upward trend in hip arthroscopy is predicted to increase into the coming decade, and anecdotally, it continues to rise.[1,5] In this article we have highlighted the major breakthroughs which led to the development of hip arthroscopy [Figure 1].
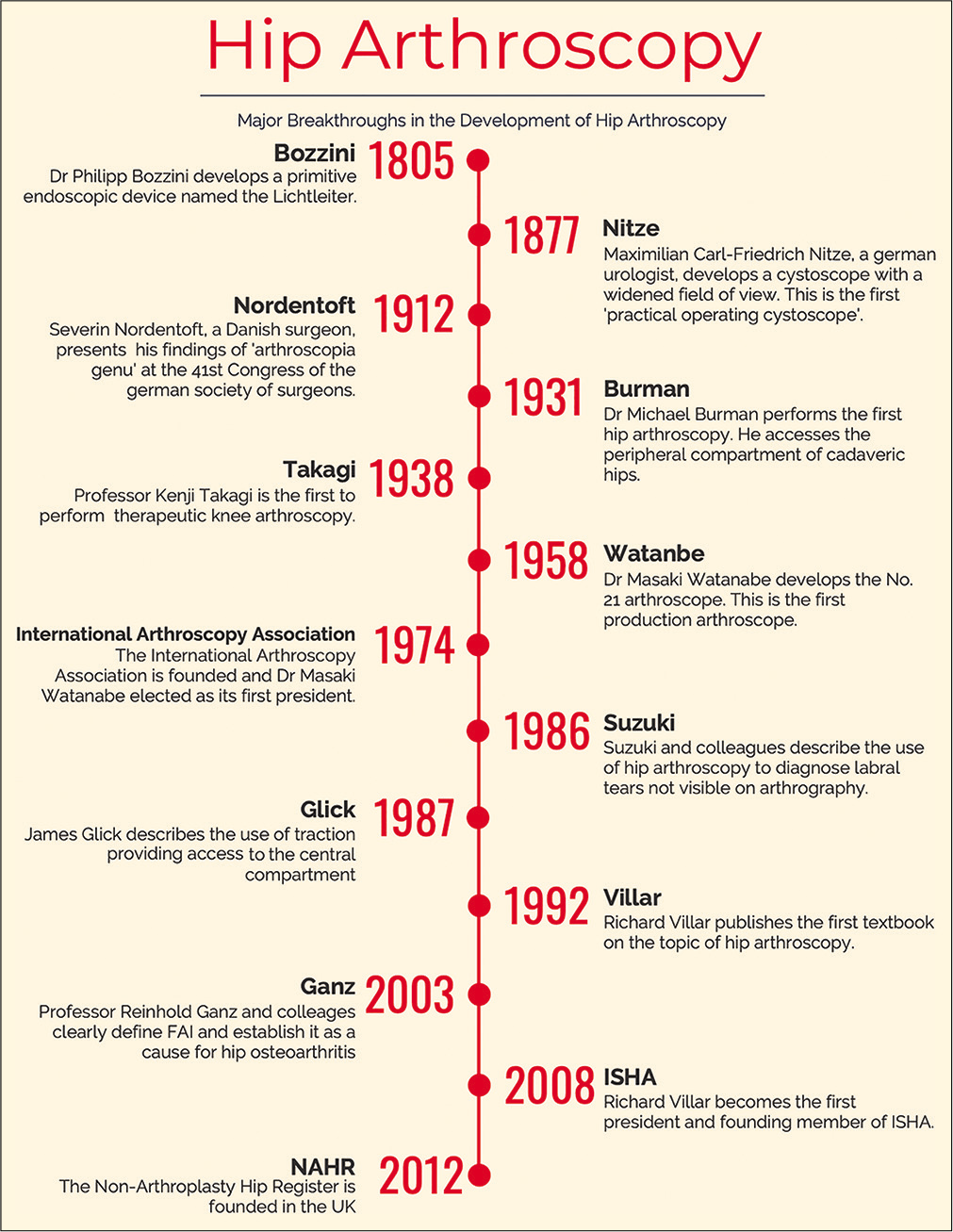
- Timeline representing the major achievements in the development of arthroscopy.
EARLY YEARS – PROBLEMS WITH ACCESS
Hip arthroscopy was first described in 1931 by Michael Burman [Figures 2 and 3], however, its widespread adoption was only achieved some 60 years later during the 1990s.[6,7] The hip joint is a deep-seated joint, with limited maneuvrability, due to high congruency within the joint. These features of the hip which provide inherent stability actually hinder arthroscopic visualization. Thus, widespread implementation of this technique was only possible once technical advances allowed adequate distraction of the femoral head from the acetabulum, providing sufficient access for arthroscopic access to the hip joint.[8] The characteristics of the hip precluded it from early investigation by endoscopy, which was developed to gain internal visualization of other body cavities including the respiratory, urinary, and gynecological tracts. These endoscopes were then adapted for the development of arthroscopy during the 20th century.
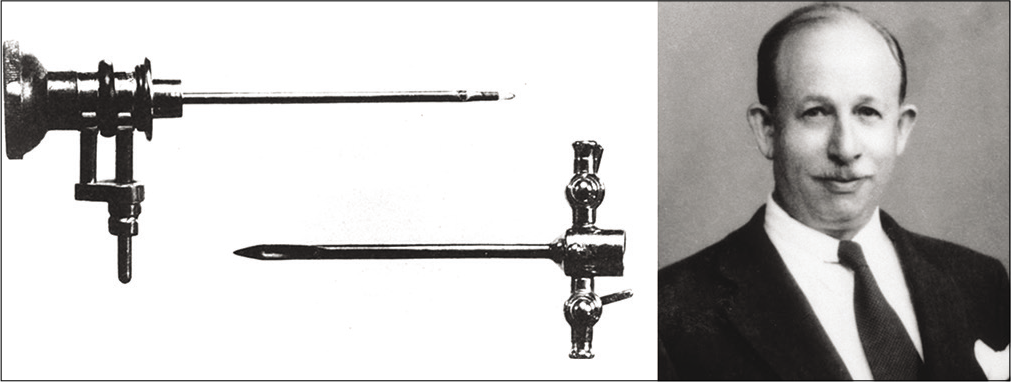
- Dr. Michael Burman and his arthroscopy equipment. The upper portion is the telescope (3 mm diameter), and lower portion is the trochar sheath (4 mm diameter). Source: Byrd 2013 – Springer “Overview and History of Hip Arthroscopy” in book “Operative Hip Arthroscopy” ISBN 978-1-4419-7925-4.
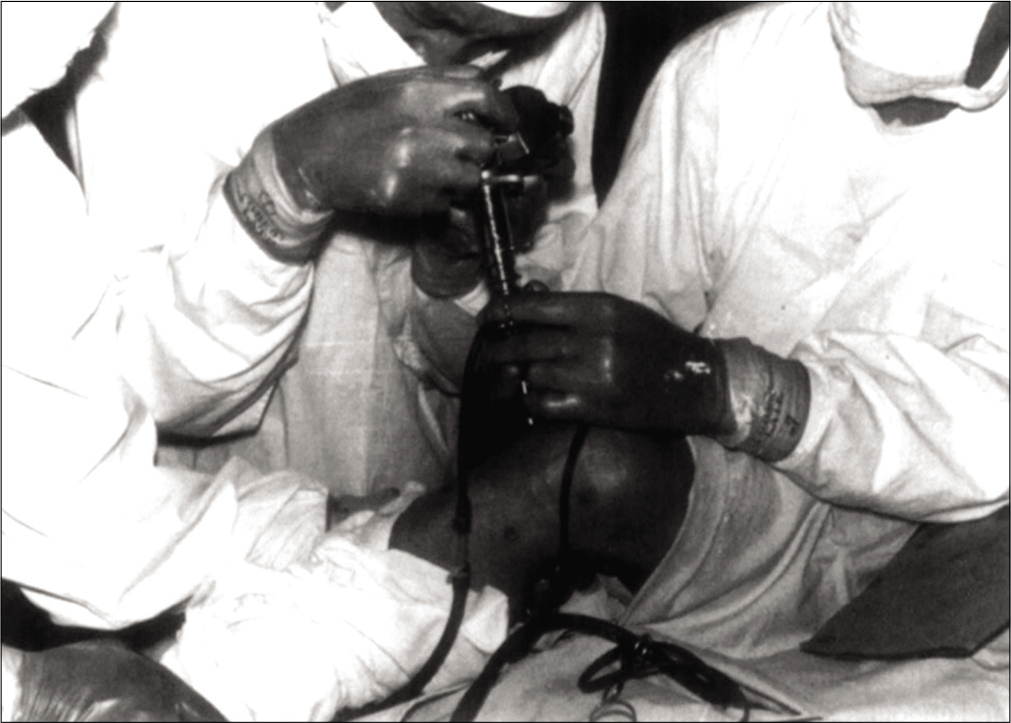
- Dr. Burman performing an arthroscopic procedure at the Hospital for Joint Diseases in 1935. Source: Byrd 2013– Springer “Overview and History of Hip Arthroscopy” in book “Operative Hip Arthroscopy” ISBN 978-1-4419-7925-4.
HISTORY
The earliest endoscopic efforts are detailed in archaeological records from Ancient Rome, detailing optimal lighting conditions and patient positioning. However, it is Dr. Phillip Bozzini who is credited as the founding father of modern- day endoscopy.[9] In the early 19th century, the Italian physician developed the “Lichtleiter” [Figure 4]. It consisted of a wax candle held by springs fortified with a system of double aluminum tubes and mirrors to allow separate lighting and imaging. This demonstrated adequate lighting and penetration through the use of different light and reflection conduits for different cavities. The clinical use of the Lichtleiter is heavily debated, with some sources claiming it was only ever used to examine canine bladders.[10] Other accounts report successful use by physicians in diagnosing pathologies including anal fistulas and vaginal canal lesions and that it was utilized in military hospitals after presentation to the Vienna-Joseph’s Academy in 1805.[9]
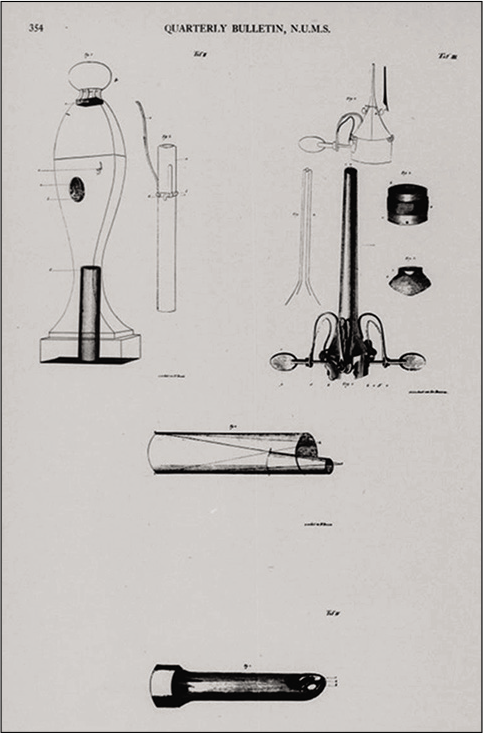
- Philipp Bozzini’s original drawing of the Lichtleiter. Source: Pearlman S 1949 “Bozzini’s classical treatise on endoscopy: A translation.” In Quarterly Bulletin of Northwestern University Medical School 23:332–354.
Bozzini’s contribution was the first of its kind and paved the way for continual endoscopic improvements during the 19th century. Similar endoscopes were designed to inspect the vagina, bladder, and urethra some 20 years later by both the French physician Segalas and the American physician Fisher.[9] These devices afforded improvements in the light sources and optics of these primitive endoscopes.
After various iterations, primitive endoscopes had been developed for visualization of internal anatomy, and numerous cystoscopic lithotripsy case reports emerged. A turning point was reached in 1853 when Désormeaux presented an endoscopic device to the Academy of Science in Paris, which provided improved internal illumination through the use of a gasogene lamp, using a mixture of alcohol and turpentine.[11] This device enabled the first reported endoscopic therapeutic surgical procedures to be performed, including endoscopic excision of a urethral papilloma. Iterative improvements in endoscope design continued, providing the basis for improvements in surgical technique as direct visualization of anatomy during endoscopic surgical excision became possible.
The year 1877 marked the beginning of modern endoscopy as Maximilian Carl-Friedrich Nitze, a German urologist, presented a cystoscope which utilized an electrified light source and a wide-angle lens.[11] The improved field of view enabled visualization of anatomy outside of the aperture of the cystoscope. Nitze reported 150 cases of cystoscopic bladder tumor removals, marking the development of the first practical operating cystoscope. These cystoscopes, and subsequent designs, were realized for their potential and used for a variety of exploratory and surgical procedures beyond their original scope. These included, for example, exploration of the pleural (thoracoscopy) and peritoneal (laparoscopy) cavities pioneered by Jacobaeus.[12] It would be over half a century until this technology would be applied to the visualization of joint cavities: Arthroscopy.
Efforts to translate endoscopy to arthroscopy were made by two pioneering surgeons, The Dane Severin Nordentoft, and the innovative Japanese professor of surgery Kenji Takagi. Nordentoft influential/widely recognized christened the term “arthroscopy” during his presentation to the 41st Congress of the German Society of Surgeons in 1912 and proposed this technique “arthroscopia genu” to visualize meniscal lesions.[13] Nordentoft, however, did not continue his work in knee arthroscopy. In 1918, Takagi [Figure 5] began his efforts using a small cystoscope to visualize the knee joint in cadavers.[14] This research work was conducted at a time where TB inflicted devastatingly high mortality and morbidity. A tuberculous knee usually resulted in ankylosed or stiff knee, conferring social and physical disability in a society where kneeling and bowing had an important cultural significance. In his quest for early detection of tuberculous arthritis, he is typically credited as being the first to advocate knee arthroscopy. Takagi sequentially developed more refined arthroscope, reducing the diameter from 7.3 mm to 2.7 mm over the span of 11 models.[15]

- Professor Kenji Takagi (1888–1963) pioneered the development of arthroscopy in Japan. Source: The Asahi Picture News - May 13, 1953.
Takagi did not publish his findings until 1933 after presentation at the Japanese Orthopaedic Association.[16] During this period, a Swiss surgeon named Dr. Eugen Bircher utilized the Jacobaeus laparoscope for visualization of the knee joint and was the first to publish on the subject.[14] The instrument was not well suited for knee arthroscopy due to a 90° optic and dead space between the lens and the tip of the instrument. Nevertheless, he published two classic articles in 1921 and 1922 in German illustrating the procedure, which utilized nitrogen gas to distend the joint in which he observed post-traumatic arthritis and meniscal pathology.[14] Following this, American arthroscopy pioneer Burman propagated the technique in the US using forward facing optics, after a 1930 fellowship in Berlin studying endoscopy. He attempted to study almost every joint in the body using a 3 mm arthroscope in cadavers and published 4 times on the subject between 1931 and 1936.[6,17-19] His 1931 publication represents the first documented arthroscopic exploration of the hip. He performed few hip studies after noting visualization was limited to only the articular surface of the femoral head and intracapsular surface of the femoral neck – the peripheral compartment described in modern hip arthroscopy. He further noted that the hip joint should be examined using a thinner arthroscope than his apparatus, which had a sheath with total outside diameter of 4 mm. He postulated that it would be impossible to insert an arthroscope between the acetabulum of the pelvis and the femoral head – meaning the labrum, and ligamentum teres would not be seen.[6,20] The idea that the modern central compartment could not be accessed may have contributed to the stagnation seen in the field of hip arthroscopy, as techniques were developed for arthroscopic exploration of the knee in the following half century.
Takagi produced the first colorized images of the inside of a knee joint in 1936 and performed the first therapeutic arthroscopic treatments of Charcot’s joints, tuberculous, and infective arthritis. He detailed these achievements in his 1938 presentation to the Japanese Orthopaedic Association, discussing clinical details of 57 cases.[20,21] It was not until WWII ended that there was a resurgence in arthroscopy progress. A Japanese surgeon and former student of Takagi, Masaki Watanabe, continued Takagi’s work in arthroscope development. He produced 24 further models and performed his first surgical procedure under arthroscopic control in 1955: Removal of a xanthomatous giant cell tumor under arthroscopic control.[14] He went on to publish an “Atlas of Arthroscopy” in 1957, and his No. 21 arthroscope [Figure 6], developed in 1958, became the first production arthroscope.[14,20] Dr. Watanabe presented his color movie “arthroscopy” at the seventh International Society of Orthopaedics Surgery and Traumatology (SICOT) congress in 1957 and subsequently at many centers in Europe and North America. Despite a good reception, arthroscopy did not receive high uptake after Watanabe’s tour of the West, and he received no communication regarding arthroscopic practice.[20] Watanabe has subsequently been credited with the development of modern arthroscopy for this work and continued contributions to the field.
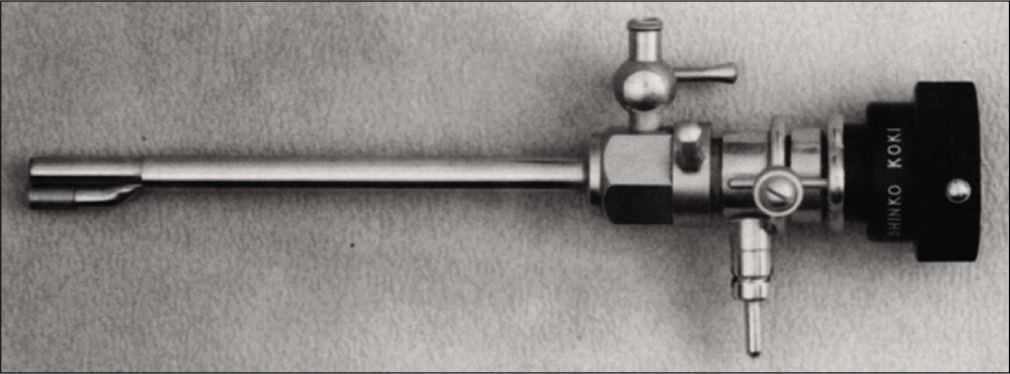
- No. 21 arthroscope. Source: Watanabe M 1986 “Memories of the early days of arthroscopy.” In Arthroscopy 2(4):209-214.
Seven years later, Dr. Robert Jackson traveled to Japan as part of a scholarship to study tissue culture at Tokyo University.[14] He took great interest in Watanabe’s work and spent time training under him. Upon Jackson’s return to Toronto, Canada, he was invigorated with the possibilities of the No. 21 arthroscope which he bought on his return.[20] He first formally presented his arthroscopy practice in 1967, at the inaugural meeting of the Association of Academic Surgeons held in Toronto. From 1968 to 1972, Jackson instructed many courses in arthroscopic surgery at the American Academy of Orthopaedic Surgeons, which proved successful. Some of his students went on to pioneer arthroscopic techniques across the country, including Dr. Ward Casscells and Dr. Jack McGinty.[14] Meanwhile in Europe, the popularity of arthroscopy was also rising. In 1975, Harold Eikelaar published his thesis on arthroscopy of the knee in Holland, and 1 year later, the first English monograph on the subject of knee arthroscopy was published.[22,23]
A new spread of awareness manifested with the foundation of the International Arthroscopy Association in 1974. Watanabe was elected as its first president. It served as a body to educate orthopedic surgeons and a number of learning courses were developed under its wing.[14] In the late 1970 and 1980s, a few prominent surgeons spearheaded the progression of hip arthroscopy. This came after labral tears were recognized as a cause of disabling hip pain and were suggested to be part of the degenerative process in 1977.[24] Subsequently, in 1986, Suzuki et al. documented the use of arthroscopy to aid the diagnosis of acetabular labral tears not visible by arthrography.[25] Progression in surgical techniques and specialized equipment then ensued.
James Glick, a surgeon in San Francisco, carried out a substantial amount of work to progress surgical technique. He published an article in 1987 detailing the insertion of an arthroscope into the hip joint through the lateral approach while the ipsilateral hip is abducted and flexed with traction being exerted appropriately.[8] The use of distraction was a significant development in the utility of hip arthroscopy and paved the way for future increase in the procedure through enabling visualization of the central compartment. In the mid-1980s, the Cambridge surgeon Richard Villar corresponded with Glick and a number of other authors who had published on the subject including Hawkins.[26] Villar published the first textbook on the topic of Hip Arthroscopy in 1992 and mentored a number of surgeons in the UK and internationally, including the senior author, with the lateral approach to hip arthroscopy.[27] Richard Villar later became the founding member and first president of the International Society for Hip Arthroscopy (ISHA) in 2008 [Figure 7].

- The founding board members of the International Society for Hip Arthroscopy, Paris, 2008. Source: Byrd 2013 – Springer “Overview and History of Hip Arthroscopy” in book “Operative Hip Arthroscopy” ISBN 978-1-4419-7925-4.
The establishment of ISHA proved to be a pivotal step in sharing knowledge around this seemingly unfamiliar procedure in the field of orthopedics, and throughout the 1990s, several manuscripts were published thereby helping advance arthroscopic surgical techniques.[28-31] Entry to the 21st century saw hip arthroscopies being performed regularly at many centers around the world. As with any health intervention, surgeons must weigh up benefits versus complications and other limitations before adopting a technique. This is especially true for hip arthroscopy which is widely regarded to have a steep learning curve.
DIAGNOSIS
Hip arthroscopy is known to aid in the diagnosis of hip disease, specifically in relation to intra-articular disease in young patients which can be a challenging area for orthopedic surgeons.[32] This is exemplified in the case of FAI and associated intra-articular pathology. FAI is a condition resulting in abnormal contact between femoral head-neck junction and acetabular rim, which may cause labral and chondral lesions in the anterosuperior region of the acetabulum due to repetitive microtrauma.[3] FAI can be classified as cam, pincer or mixed types.
While plain radiographs have poor value in the assessment of intra-articular pathology, they are still a valuable investigation for the assessment of FAI by evaluating femoral and acetabular bony abnormalities.[33] MRI remains the gold standard option as a non-invasive imaging diagnostic procedure. It has a reported sensitivity of 85–90% for labral tears and 91-92% for chondral damage.[34,35] However, in comparison to arthroscopic examination, it has a reported specificity of 70% for labral tears and 62% for chondral lesions.[34] Such findings demonstrate that invasive imaging techniques including arthroscopy and magnetic resonance arthrography (MRA) maintain a key diagnostic role for intra-articular pathology. MRA is better at identifying labral pathology, as intra-articular gadolinium contrast medium is administered. It achieves a high sensitivity and specificity for labral tear identification of 95% and 92%, respectively, and of 94% and 91%, respectively, for chondral lesions.[36] Despite the success of invasive imaging, arthroscopy still retains a diagnostic role, albeit limited, especially in the case of undiagnosed hip and groin pain where all the investigations are equivocal, and it is the gold standard test for the identification of intra-articular pathology.
TREATMENT
Hip arthroscopy is a surgical intervention used in the management of many hip-related pathologies and shows high patient satisfaction and overall success rates. In the case of FAI, arthroscopic surgical intervention can be utilized for osseous correction through femoroplasty or acetabuloplasty, and labral and chondral damage can be addressed. The osseous correction will decrease head-neck offset or reduce acetabular over coverage of the femoral head, thereby restoring hip joint function. In this setting, hip arthroscopy provides significant improvements in general and hip-related quality of life scores postoperatively in patients without advanced OA.[37] While arthroscopy still produces positive outcomes in some individuals with OA, its efficacy is reduced.[38] A more rapid progression to total hip arthroplasty is observed in those with severe chondropathy and advanced age. Such findings highlight the importance of early detection for conditions which predispose to OA, and the advances in arthroscopy has enabled in their diagnosis and management.
While hip arthroscopy has positive outcomes, a comparative overview is required when considering the efficacy of a treatment option. A number of studies contrast hip arthroscopy with traditional hip arthrotomy and conservative management. Some clinicians may prefer non-operative management which includes physiotherapy, intra-articular steroid injections, or active monitoring. These options have been outlined in the Warwick agreement regarding the management of FAI as part of an appropriate management pathway.[39] It has been shown, however, that patient-reported outcome measures (PROMs), including non- arthritic hip scores (NAHS) and modified Harris hip score, are significantly higher in FAI treated using hip arthroscopy compared with physiotherapy.[40,41] In a large multicenter RCT, hip arthroscopy showed an iHOT-33 (PROM) score 6.8 points higher than physiotherapist-led conservative care when adjusted for baseline score and patient characteristics at primary outcome assessment, representing a clinically significant difference.[41]
Hip arthroscopy outcomes are also favorable in comparison to hip arthrotomy with open surgical dislocation, which is also a surgical option for surgical management of FAI. Patients who underwent hip arthroscopy scored significantly higher NAHS at 3 and 12 months follow-up and also reported to have a lower rate of revision.[42] However, other PROMs at 12-month follow-up did not differ significantly between the procedures. This may indicate the benefits of the decreased recovery time hip arthroscopy offers. It is known that there are complications and risks associated with joint dislocation including an increase in the rate of infection, deep vein thrombosis (DVT), and avascular necrosis. Furthermore, the minimally invasive surgery approach affords reduced blood loss, soft-tissue damage, scarring, and improved post-operative capsule integrity.[43] Conversely when compared to open surgical dislocation, hip arthroscopy provided a significantly lower reduction of the alpha angle measured by the Dunn lateral view for individuals with cam deformity.[42] This is likely due to the surgeon’s assessment of the lesion using 2D modalities, including the arthroscopic appearance and fluoroscopy.[44] Navigated surgery techniques, however, are able to reduce the rate of arthroscopic under- resection.[45] While there is not total agreement in the literature, hip arthroscopy has been adopted by the majority of surgeons over open arthrotomy.
There are, however, a number of other conditions which report better outcomes when treated with open surgery versus hip arthroscopy. These include acetabular dysplasia, Legg-Calve-Perthes disease, and chronic slipped capital femoral epiphysis.[46] Such cases illustrate the importance of astute patient selection for surgery. Hip arthroscopy has many benefits over traditional hip surgery which is exemplified in the management of FAI, but it should not be considered the default procedure for every patient with intra- articular pathology.
COMPLICATIONS
Hip arthroscopy is a successful technique, although a wide range in complication rate has been reported.[47] A recent systematic review of observational studies determined the complication rate of hip arthroscopy to be 3.32%.[48] The three most common complications were neuropraxia, iatrogenic chondral and labral injury, and heterotopic ossification with rates of 0.92%, 0.69%, and 0.60%, respectively. Major complications made up 4.8% of all complications, and the most common major complication was abdominal fluid extravasation.
Emphasis is placed on the importance of patient selection to achieve favorable complication rates for hip arthroscopy. Obese, older, and dysplastic females have the worst reported post-operative outcomes from hip arthroscopy.[49] Obesity is also a known risk factor for the development of osteoarthritis, and it has now been demonstrated that there is a lower overall outcome and a higher rate of revision surgeries in the obese. Given obesity is becoming an increasingly prevalent problem worldwide, physiotherapy and weight loss may be advised before considering a surgical solution. In addition, within groups of females with FAI separated according to age and bony characteristics, older patients with borderline dysplasia had poorer iHOT-12 scores compared to other groups.[50] Such patient risk factors may guide a surgeon’s management decision.
Other non-specific complications of hip arthroscopy include DVT which has a likelihood of 4.3%.[51] However, routine thromboprophylaxis is not advocated by everyone, thereby requiring the surgeon to assess the risk of venous thromboembolism on a case-by-case basis and institute appropriate mechanical and/or chemical thromboprophylaxis. Other less frequent complications include femoral neck fractures.[52] Despite decreased complication rates in high-volume surgeons, no correlation has been shown between the number of hip arthroscopies a surgeon had performed and the rate of hip fracture as a complication.
TRAINING
The British Non-Arthroplasty Hip Register (www.nahr.co.uk) recorded a 250% increase in hip arthroscopies performed between 2007 and 2011. This increase in popularity has presented many advantages as well as some drawbacks. It is commonly acknowledged that there is a steep learning curve associated with hip arthroscopy.[53] During the learning of this procedure, there is a significantly higher complication rate in the first 30 patients, and operative time decreases as the number of arthroscopies performed increases. It has been suggested that the complication rates in low-volume surgeons are unacceptably high, which raises an ethical dilemma for the adoption of the procedure.[54] A plausible solution would be to encourage more orthopedic surgeons to adopt hip arthroscopies into their work, and for learning to occur only at specialist centers, where there is adequate supervision to optimize practice and training. There is also an emerging role for virtual reality simulation of hip arthroscopy in the training of surgeons. This has demonstrated a significant improvement in surgeon performance after three sessions.[55]
New technologies in theater prove challenging for surgeons. This is especially true for minimally invasive surgery (MIS) where there is a more demanding work environment and there are less rigorous safety and testing standards for instruments compared to similar medical industries such as pharmaceuticals.[56] Studies show that recently trained surgeons are more likely to use MIS techniques, with a gradual shift in the operative techniques utilized. Furthermore, it appears to be beneficial to invest in the training of the supporting surgical team.[57]
CONCLUSION
The journey of hip arthroscopy has been impressive and it is certainly here to stay. However, hip arthroscopy provides clear positive outcomes only when patients are correctly stratified and selected, and in the hands of an experienced operator. The future holds new exciting possibilities for hip arthroscopy both in terms of technological advances in helping better stratification of disease, accuracy of the procedure (robotics) and training (simulators), and also biological advances helping with addressing the damaged articular cartilage.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vikas Khanduja is on the Editorial Board of the Journal.
References
- Past and projected temporal trends in arthroscopic hip surgery in England between 2002 and 2013. BMJ Open Sport Exerc Med. 2016;2:e000082.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts in the diagnosis and management of extra-articular hip impingement syndromes. Int Orthop. 2017;41:1321-8.
- [CrossRef] [PubMed] [Google Scholar]
- Femoroacetabular impingement: A cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-20.
- [Google Scholar]
- Trends in utilization and outcomes of hip arthroscopy in the United States between 2005 and 2013. J Arthroplasty. 2017;32:750-5.
- [CrossRef] [PubMed] [Google Scholar]
- Femoroacetabular impingement: The past, current controversies and future perspectives. Phys Sportsmed. 2018;46:270-2.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopy or the direct visualization of joints. Bone Joint Surg. 1931;13:669-94.
- [Google Scholar]
- Historical review of arthroscopic surgery of the hip. Int Orthop. 2017;41:1983-94.
- [CrossRef] [PubMed] [Google Scholar]
- (1773-1809): The earliest description of endoscopy. J Med Biogr. 2018;26:137-41.
- [CrossRef] [PubMed] [Google Scholar]
- The Evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-7.
- [Google Scholar]
- Nezhat's History of Endoscopy: A Historical Analysis of Endoscopy's Ascension Since Antiquity (1st ed). Tuttlingen: Endo-Press; 2005. p. :196.
- [Google Scholar]
- Severin nordentoft: The first arthroscopist. Arthroscopy. 2001;17:532-5.
- [CrossRef] [PubMed] [Google Scholar]
- From the scalpel to the scope: The history of arthroscopy. Proc (Bayl Univ Med Cent). 1996;9:77-9.
- [CrossRef] [Google Scholar]
- McGlamry's Comprehensive Textbook of Foot and Ankle Surgery (2nd ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2017. p. :2112.
- [Google Scholar]
- Acetabular labrum tears: A cause of hip pain and degenerative arthritis. South Med J. 1977;70:174-5.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic diagnosis of ruptured acetabular labrum. Acta Orthop Scand. 1986;57:513-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10:275-80.
- [CrossRef] [Google Scholar]
- Hip arthroscopy: An anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11:418-23.
- [CrossRef] [Google Scholar]
- Arthroscopy of the hip: 12 years of experience. Arthroscopy. 1999;15:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy without traction: In vivo anatomy of the peripheral hip joint cavity. Arthroscopy. 2001;17:924-31.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding painful hip in young adults: A review article. Hip Pelvis. 2019;31:129.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res. 2011;469:464-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hip pathology: The diagnostic accuracy of magnetic resonance imaging. J Orthop Surg Res. 2018;13:127.
- [CrossRef] [PubMed] [Google Scholar]
- Acetabular labral tears and cartilage lesions of the hip: Indirect MR arthrographic correlation with arthroscopy-a preliminary study. AJR Am J Roentgenol. 2010;194:709-14.
- [CrossRef] [PubMed] [Google Scholar]
- MR arthrography versus conventional MRI in evaluation of labral and chondral lesions in different types of femoroacetabular impingement. Egypt J Radiol Nucl Med. 2017;48:169-78.
- [CrossRef] [Google Scholar]
- Health-related quality of life after hip arthroscopy for femoroacetabular impingement: A systematic review and meta-analysis. Sports Health. 2019;11:209-17.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy in the setting of hip osteoarthritis: Systematic review of outcomes and progression to hip arthroplasty. Clin Orthop Relat Res. 2015;473:1055-73.
- [CrossRef] [PubMed] [Google Scholar]
- The Warwick agreement on femoroacetabular impingement syndrome (FAI syndrome): An international consensus statement. Br J Sports Med. 2016;50:1169-76.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic hip surgery compared with physiotherapy and activity modification for the treatment of symptomatic femoroacetabular impingement: Multicentre randomised controlled trial. BMJ. 2019;364:185.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): A multicentre randomised controlled trial. Lancet. 2018;391:2225-35.
- [CrossRef] [Google Scholar]
- Hip arthroscopy versus open surgical dislocation for femoroacetabular impingement: A systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e5122.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy for the management of trauma: a literature review. J Hip Preserv Surg. 2015;2:242-8.
- [CrossRef] [PubMed] [Google Scholar]
- Review: Current concepts in computer-assisted hip arthroscopy. Int J Med Robot Comput Assist Surg. 2018;14:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of navigated cam resection in femoroacetabular impingement: A randomised controlled trial. Int J Med Robot Comput Assist Surg. 2017;13:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Techniques and results for open hip preservation. Front Surg. 2015;2:64.
- [CrossRef] [PubMed] [Google Scholar]
- Complications in hip arthroscopy. Muscles Ligaments Tendons J. 2016;6:402-9.
- [CrossRef] [PubMed] [Google Scholar]
- Complications following arthroscopic surgery of the hip: A systematic review of 36 761 cases. Bone Joint J. 2017;99:1577-83.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy in obese, a successful combination? J Hip Preserv Surg. 2015;3:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Trajectory of clinical outcomes following hip arthroscopy in female subgroup populations. J Hip Preserv Surg. 2019;6:25-32.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence of proximal deep vein thrombosis after elective hip arthroscopy: A prospective cohort study in low risk patients. J Hip Preserv Surg. 2016;3:295-303.
- [CrossRef] [PubMed] [Google Scholar]
- Femoral neck fractures as a complication of hip arthroscopy: A systematic review. J Hip Preserv Surg. 2017;4:9-17.
- [CrossRef] [PubMed] [Google Scholar]
- Hip arthroscopy: Analysis of a single surgeon's learning experience. J Bone Joint Surg Am. 2011;93(Suppl 2):52-6.
- [CrossRef] [PubMed] [Google Scholar]
- What is the role of minimally invasive surgery in a fast track hip and knee replacement pathway? Ann R Coll Surg Engl. 2012;94:148-51.
- [CrossRef] [PubMed] [Google Scholar]
- The learning curves of a validated virtual reality hip arthroscopy simulator. Arch Orthop Trauma Surg. 2020;140:761-7.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of risk factors in minimally invasive surgery: A prospective multicenter study. Surg Endosc. 2017;31:2467-73.
- [CrossRef] [PubMed] [Google Scholar]
- Training young adult hip surgeons for the future: The Cambridge vision. Bone Jonit 360. 2016;5:8-12.
- [CrossRef] [Google Scholar]







