Translate this page into:
Recent advances and future trends in wrist arthroscopy

-
Received: ,
Accepted: ,
How to cite this article: Viswanath A, Talwalkar S. Recent advances and future trends in wrist arthroscopy. J Arthrosc Surg Sport Med 2020;1(1):65-72.
Abstract
For a long time, wrist arthroscopy has languished behind that of shoulder and elbow arthroscopy. However, over the past two decades, there has been a steady increase in therapeutic wrist procedures undertaken using the arthroscope. While diagnostic wrist arthroscopy is still a useful tool, its therapeutic advantages are starting to stack up against the risks of open wrist surgery – mainly stiffness. It remains a technically demanding procedure, but is clearly in the armamentarium of orthopedic hand and wrist surgeons. Recent advances of dry arthroscopy, arthroscopic reduction and internal fixation, and arthroscopic fusion procedures have changed the face of minimally invasive wrist surgery. The new NanoScope™ along with wide-awake, local anesthetic, and no tourniquet techniques, means that we now can dynamically assess and treat wrist pathology without even encountering the risk of anesthesia. Wrist surgery is evolving, and arthroscopy is right at the forefront.
Keywords
NanoScope
Dry arthroscopy
Recent advances
Wrist arthroscopy
INTRODUCTION
Arthroscopy was first introduced in the early part of the 20th century,[1] but it was not until the 1970s that its clinical application was widely undertaken. As knee arthroscopy was starting to become a routine tool for orthopedic surgeons, Chen described the use of the arthroscope for examining the wrist in 1979.[2] By the mid-1980s, wrist arthroscopy was being used in larger orthopedic units, aided by the instructional lecture in the USA by Roth et al.[3] It has now become part of the armamentarium of the hand and wrist surgeon.
Early publications of the first series of wrist arthroscopies focused on its use as a diagnostic tool[4,5] and the realization that extrinsic ligaments could be appreciated far more easily. It became another rung in the diagnostic ladder of the persistently painful wrist. With reports of it being more sensitive compared with arthrography,[6] it has now become the gold standard for evaluating chronic wrist pain. Slowly, the indications for wrist arthroscopy were starting to emerge. Classifying wrist pain as extra- or intra-articular would be crucial in determining the indication for arthroscopic evaluation.[7] Typical portal sites to be used were clearly defined and the patient setup became largely standardized with some form of traction tower. The procedure is technically demanding, however, and complications mainly related to sensory nerve injury were recognized.[8] Nevertheless, the benefits of arthroscopic wrist surgery were huge in comparison to open surgery, where arthrofibrosis can be a massive drawback.[9]
The use of wrist arthroscopy has allowed us to understand the complexity of the anatomy of the TFCC. By 1996, an arthroscopic classification system of scapholunate (SL) ligament injuries emerged[10] and the complex anatomy of radial-sided ligamentous stabilizers was appreciated. With these soft-tissue lesions now being assessed arthroscopically, it was only a matter of time before the therapeutic options tumbled in. By 2008, 87% of wrist arthroscopies by high- volume wrist surgeons were therapeutic.[11]
For many years, advances in wrist arthroscopy languished behind its proximal neighbors, with elbow and shoulder arthroscopic techniques evolving rapidly. The primary issue seemed to be the small working and the wrist’s envelopment by tendinous and neurovascular structures. Although the use of arthroscopy as a diagnostic tool in the knee and shoulder fell away, it was for a long time the major indication in the wrist.[12] Moreover, while wrist arthroscopy may have discreetly lagged behind that of other joints, new innovation in nanotechnology proves that the future of wrist arthroscopy is anything but quiet.
In this review article, we give an overview of the equipment needed, outline the advantages of wrist arthroscopy – both diagnostic and therapeutic, and look to recent trends and future prospects on the horizon.
EQUIPMENT AND SETUP
Although there are some variations in set-up, the basic premise is largely the same. In our institution, we have the patient supine on the operating table, the shoulder is abducted and the elbow flexed to 90°. The hand and forearm are prepped with aqueous chlorhexidine, and two or three digits are placed in sterile fingertraps attached to the traction device. A 5 kg counter weight is hung over the arm, whether a tourniquet is used or not [Figure 1a]. This set-up is adequate for a short procedure, but if the complexity of the case increases and demands more surgical time, a traction tower (such as the acumed arc wrist tower) is required to prevent pressure issues due to the countertraction weight [Figure 1b].
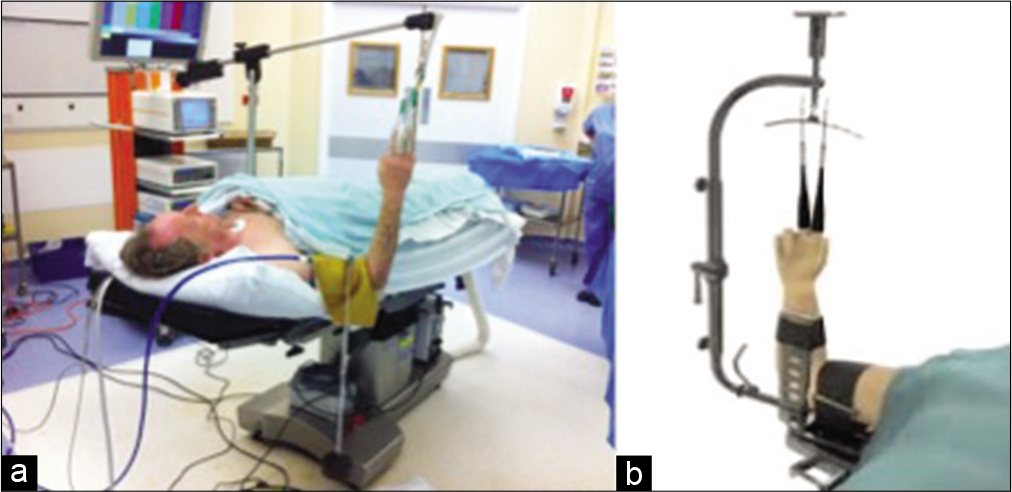
- The setup of wrist arthroscopy. (a) Standard set-up for a diagnostic scope using a counterweight over the arm. (b) Traction tower device – here the Acumed Arc Wrist Tower is shown.
The arthroscope itself can be between 1.7 and 3.0 mm, but usually we utilize the 2.7 mm scope with a 30° viewing angle and an angled 3 mm probe. The irrigation system has a gravity-dependent inflow and manual outflow, with a fluid pump to allow pressures of 30–40 mmHg.
The portals have been well established[5] and the portals are named for the extensor compartment interval they utilize. The main and first portal to create is the 3–4 portal, which allows access to the radiocarpal joint [Figure 2]. The skin is carefully incised through the dermis only, and then, blunt dissection generates the portal itself. The “box concept” proved that viewing and working portals could encircle the whole wrist.[13] In theory, this could allow an infinite number of other portals to be made as long as a good working knowledge of wrist anatomy is borne in mind.
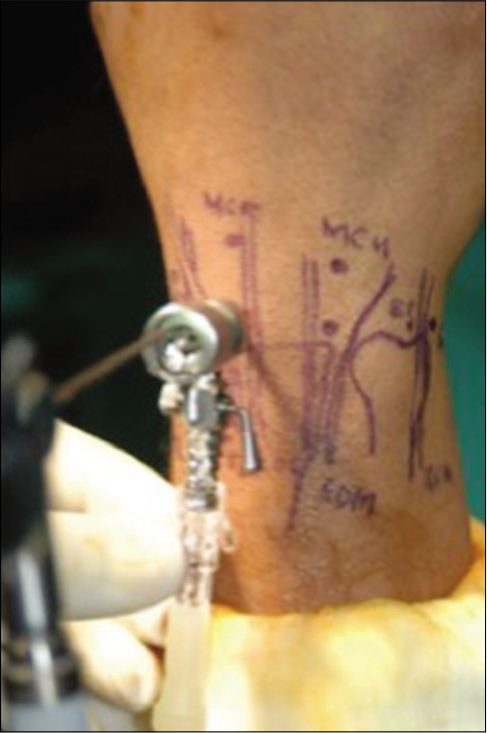
- Dorsum of the wrist showing established portal markings and the 3–4 portal with scope inserted.
Volar portals have been described, but have not gained widespread use. One of the more useful volar portals is the volar radial (or FCR) portal, popularized by Slutsky.[14] This portal is made through the bed of the FCR tendon at the level of the proximal wrist crease. It has been described as a safe zone, but the palmar cutaneous branch of the median nerve is close, although nearly always lies on the ulnar side of the FCR tendon. This portal is particularly useful in assessing the dorsal radiocarpal ligament.
The volar central portal, as described by Corella et al.,[15] is established through a 1.5 cm incision from distal to proximal wrist crease in line with the third intermetacarpal space [Figure 3]. The mid-carpal portal is developed distal to the anterior horn of the lunate through the space of Poirier and allows access to the entire mid-carpal joint. The advantage of this entry is the reduced risk of damage to the scaphocapitate or triquetrohamocapitate ligaments. Such a portal is beneficial to use when undertaking advanced procedures such as SL ligamentoplasty, arthroscopic proximal row carpectomy (PRC), or arthroscopic limited arthrodesis.
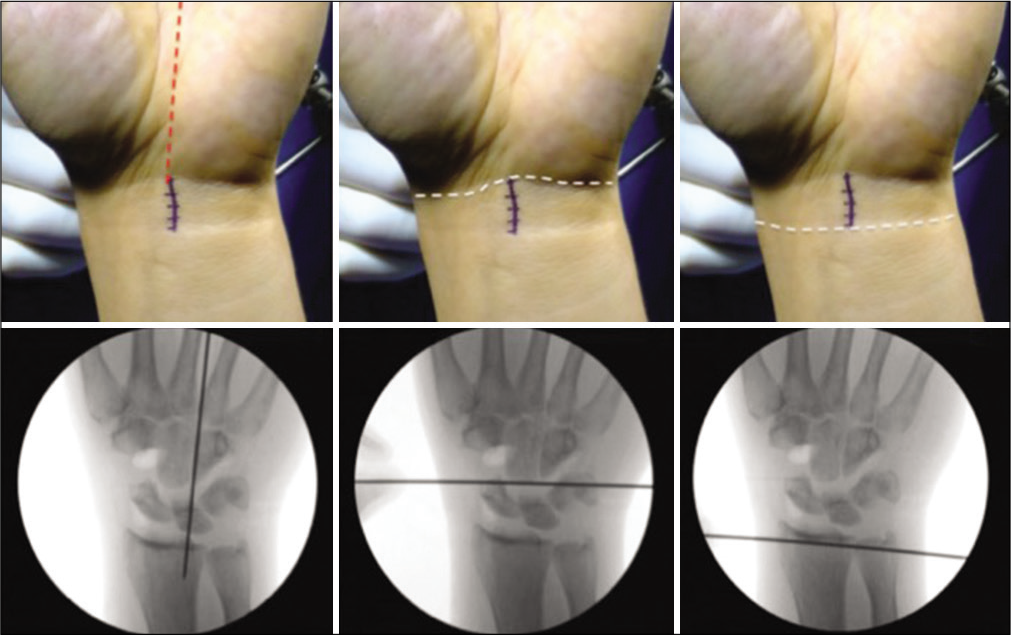
- The volar central portal as described by Fernando Corella. A 1.5 cm incision made from distal to proximal wrist crease in line with the 3rd intermetacarpal space, with corresponding radiographs shown below each landmark (reprinted with permission from F Corella).
ADVANTAGES OF ARTHROSCOPIC EXAMINATION
The diagnostic value of wrist arthroscopy has been clearly documented,[16] particularly when evaluating a persistently painful wrist likely to be intra-articular in origin. The best candidates for wrist arthroscopy tend to be those with mechanical wrist pain,[17] as TFCC injuries, ligamentous injuries, and cartilaginous lesions can be readily picked up.
TFCC injuries
Asymptomatic TFCC tears are not uncommon, so correlation with a thorough history and examination is vital. Arthroscopic evaluation of the TFCC has been described in many ways,[18-20] all of which test the tension in the ligamentous complex. It, therefore, became possible to classify the injury as per location and reparability. For distal tears, many suturing techniques have been described[21] and arthroscopic treatment is now the mainstay. Clinical outcomes between open and arthroscopic TFCC repair show no statistical differences,[22] and patient-reported outcomes are also satisfactory.[23] For proximal tears or where reattachment to the fovea is necessitated, the options include arthroscopic or open surgery. Arthroscopic assessment allows visualization of the distal radioulnar joint (DRUJ) and potential instability, and if present, whether there is concomitant arthritis. This becomes crucial in determining the next phase of treatment as reconstruction or arthroplasty.
Sports injuries
Athletes pose a treatment dilemma for orthopedic surgeons: They generally are not candidates for activity modification. Their marker of a successful procedure is their ability to continue with professional sport at their pre-injury level. Arthroscopy provides a less aggressive approach to treat wrist injuries and may allow a quicker recovery.[24] In a retrospective study, 13 professional tennis players (WTA and ATP ranking) had wrist arthroscopy after an average career length of 8.4 y.[25] By their post-operative year 2, there was no significant decline ranking compared with pre-injury. The average time to return to professional tennis was 148 days. Compared with the hip, shoulder, and elbow, wrist arthroscopy saw the smallest decline in ranking.
In a military population, wrist pain can adversely affect push-up performance. A retrospective review of arthroscopic thermal shrinkage used for SL ligament instability showed a statistically significant reduction in pain and 86% rate of return to active duty.[26] Even chronic SL tears treated arthroscopically have allowed professional athletes to return to their previous level of play.[27] While the sample sizes are small, these studies show that arthroscopic intervention gives good outcomes with a much quicker recovery period in high- demand adults.
Staging and operative planning
One of the key advantages of arthroscopy is the ability to clearly delineate the stage of cartilage degeneration. It allows for direct visualization of softening, tears, and fibrillation, which may otherwise be missed or underestimated by MR imaging as the cartilage thickness is so much less in the carpus than in the knee.[28] This is slightly different from purely a diagnostic arthroscopy, as it allows for a degree of debridement as well as assessment. Knowing the extent of chondral defects enables a more confident treatment plan, such as progressing to PRC in those with no capitate head lesions. This may now be undertaken in an arthroscopic or open manner.
Keinböck’s disease is another condition where probing the lunate for softening or chondral flaps dictates the best treatment option. The Bain and Durrant arthroscopic classification system[29] uses arthroscopic findings to direct surgical options to maintain functional motion. Even where there is no articular compromise, the value of arthroscopy is the ability decompress through arthroscopic forage once no cartilage defect is noted.[30] In a similar way, wrist arthroscopy is beneficial in Preisser’s disease also.
CURRENT TREATMENTS PERFORMED BY ARTHROSCOPY
As with most arthroscopic procedures, the initial therapeutic interventions were an extension of the diagnostic procedure, such as joint debridement and irrigation, even in septic joints. As instrumentation became more familiar, loose body removal, synovectomy, and synovial biopsy were all also performed. Below are some of the more common arthroscopic procedures currently widely performed on the wrist.
TFCC repair
The TFCC was really only fully anatomically appreciated after wrist arthroscopy became more commonplace. TFCC debridements were one of the first few therapeutic procedures to be undertaken arthroscopically[31] and that has now progressed to TFCC repairs – mainly of distal tears. Atzei described arthroscopic-assisted proximal TFCC tear repairs and reattachment to the fovea in 2009.[21]
In 2011, Atzei and Luchetti devised a comprehensive classification of peripheral TFCC tears based on clinical, radiographic, and arthroscopic assessment.[32] The assessment consists of radiocarpal arthroscopy to visually confirm a peripheral tear, its size and tissue quality, and then a hook test to check tautness of its proximal insertion. They suggest foveal refixation for Class 2 and 3 tears, styloid fixation for Class 3A tears, and tendon graft reconstruction for Class 4 tears. More recently, an all-arthroscopic tendon graft reconstruction for chronic DRUJ instability has been described.[33] This technique is based on the Adams-Berger reconstruction, but modifies the tunnels to allow an all- arthroscopic procedure. The proponents of this technique suggest that it reduces ulnar-sided nerve injuries and rigidity by avoiding an arthrotomy.[33]
In combination with a TFCC procedure, the ulnar variance can readily be assessed. Ulnar abutment or impingement can be directly visualized through dynamic assessment. Whilst ulnar shortening is an extra-articular procedure, the arthroscopic wafer procedure allows for intra-articular treatment without the need for hardware. This overcomes the issues of hardware prominence and circumvents non-union rates of about 10%, while also allowing for a quicker return to work.[34]
SL ligament injuries
The radial-sided ligament complex has now been explored more thoroughly and a new concept of the SL complex is emerging[35] with dorsal, volar, and proximal subregions. Arthroscopic dorsal ligamentous repair with or without associated pinning in the dynamic instability is now considered the less damaging and denervating than open repair.[35] In cases of pre-dynamic instability, Belyea et al. have shown that thermal shrinkage can be very effective with a quick return to work in the young, active population.[26] Even long-term follow-up studies have shown proven benefit in the low-grade Geissler SL ligament tears treated with thermal shrinking.[36] This has the added benefit of denervation of the ligament, which may reduce post-operative pain. Unlike in the knee or shoulder, where thermal shrinkage for instability is now rarely used, the wrist can be fully immobilized after the procedure to allow the full effect of the treatment through fibroblastic nuclear pyknosis.[37]
In symptomatic patients with a complete SL ligament tear and with easily reducible instability, Corella et al. have pioneered a dorsal and volar all-arthroscopic ligament reconstruction technique.[38,39] The 3 mm FCR graft is fixed to the scaphoid and lunate using interference screws, and early mobilization is encouraged. The technique also utilizes both dorsal central and volar central incisions for portal access [Figure 4]. In their series of 27 carefully selected patients, they show preserved wrist range of motion, improvement in visual analogue scores, and improvement in grip strength, all with an early post-operative range of movement rehab protocol.[38]

- All arthroscopic scapholunate ligament reconstruction using FCR graft, with interposition screws in scaphoid and lunate bone tunnels. When the graft is passed through the scaphoid tunnel and tensioned, the scaphoid extends. When the graft is passed through the lunate tunnel, the scaphoid supinates. Volar fixation prevents volar opening and movement in the sagittal plane (reprinted with permission from F Corella).
Ganglionectomy
From the mid-90s, dorsal ganglia of the wrist has been excised arthroscopically with excellent results.[40] The main advantage is that the stalk can often be seen and excised primarily at the time of ganglionectomy, and even if not seen, the capsule can be debrided near the dorsal SL ligament. The technique has now been adapted for volar wrist ganglia[41] and has been performed using the dry arthroscopy technique too[42] showing an earlier return to function.
Arthrolysis
One of the big advantages of wrist arthroscopy is the reduction in rates of stiffness as compared with open surgery. Stiffness of the wrist can also occur following trauma. This can be treated with arthroscopic arthrolysis with minimal complications and a reduction in pain score.[43] The surgical procedure involves resection of intra-articular adhesions, release of certain ligamentous structures, and capsulotomy, all of which can be technically demanding and time consuming, but warrants good results[44] with an improvement in flexion- extension of a mean of 15°. In fact, the technique has recently been used in a case of pediatric arthrogryposis utilizing radiocarpal and mid-carpal portals.[45]
RECENT ADVANCES IN ARTHROSCOPIC MANAGEMENT
Arthrodesis
Limited arthrodesis can be carried out in an arthroscopic manner, although it is technically very demanding and has a steep learning curve. The operative time has been noted to be long, and fixation options are limited to K-wires or cannulated screws, and in addition, the complications have not been insignificant, with prominent metalware and tendon rupture noted.[46] However, the procedure is in its infancy and it is yet to be determined as to whether it confers benefit over the traditional open approach.
PRC
The excision of the scaphoid, lunate, and triquetrum to enable the lunate fossa to articulate with the head of the capitate is a procedure, which has now been performed arthroscopically in larger centers.[47] Importantly, the state of the articular cartilage of the capitate must be thoroughly inspected before undertaking this operation, which utilizes multiple working portals to excise the entire row. Hernandez et al. have, however, used the volar central portal to undertake this procedure[48] to avoid damage to the posterior interosseous nerve. The advantages of performing this procedure arthroscopically, for carpal arthritis and for Kienböck’s disease, include less soft-tissue detachments, earlier mobilization and less post-operative stiffness.
Arthroscopic-assisted fracture reductions
Several studies have identified an intra-articular distal radial fracture without a large extra-articular component, and with 1–2 mm of articular incongruency after closed reduction, as the fracture pattern indicated for arthroscopic- assisted treatment.[49] The articular surface can be clearly seen and temporary plate fixation can be adjusted until true congruency is achieved. The technique is truly “assisted” in that the procedure begins with an open reduction and plate applied in the usual manner, but the forearm position is then changed for the arthroscopic set-up and definitive fixation occurs in this new position after confirming anatomic reduction. To date, no study has been able to correlate superior fixation of this technique with functional outcomes. The advantage of arthroscopic-assisted cases, however, is the recognition of associated injuries such as SL ligament or TFCC tears.
Perilunate injuries, not dislocated have recently been described as being treated by the arthroscopic-assisted method[50] with a quick time to union in the one case presented; and scaphoid non-unions have also been treated using this method with the added advantage if being able to probe the fracture site and debride fibrous tissue as needed.[51]
Dry arthroscopy
When considering using arthroscopy for fracture reduction, a fluid-filled field causes disruption and can be detrimental. Del Pinal et al. has expounded the virtues of dry arthroscopy with excellent results in over 100 cases.[52] Although risks of wrist arthroscopy are low, the use of a dry field further reduces the risk of fluid extravasation and therefore compartment syndrome. It also allows an easier conversion to open surgery. In our institution, all diagnostic scopes are dry arthroscopies, with the ability to turn on the irrigation as needed for the therapeutic procedure. This in itself gives less swelling and, therefore, less pain.
With arthroscopic-assisted fracture fixation, the dry scope allows better assessment through less disruption due to fluid flow; however, the view is often less than perfect. The view can be improved by cleaning the scope intra-articularly on nearby fat or fascia, and spinal pledgelets (surgical patties) can be used to “mop up” blood and excess fluid in the field. Small injections of fluid can also be introduced to the field, providing in essence, a moist field, rather than a fully dry one.
Even in soft-tissue procedures such as ganglionectomy, the dry arthroscopy technique has been associated with a lower complication rate[42] with no post-operative refilling of the ganglion “pocket.”
Bone grafting
Scaphoid non-unions can be successfully treated with distal radius bone grafting and fixation. This has now been described arthroscopically in a case series of 16 patients in Denmark.[53] Of the 16, eight were treated in a standard minimally invasive manner and eight by arthroscopic bone graft and screw. The arthroscopic bone graft group healed significantly faster than the percutaneous group. This again is a new technique using dry arthroscopy with intermittent saline infiltration to aid visual field. As per Del Pinal et al., the use of dry arthroscopy in such cases requires adjustment of technique using fat of soft-tissue structures to clean off the scope tip and by intermittent irrigation and surgical patty use.[52]
FUTURE TRENDS OF WRIST ARTHROSCOPY
Wide-awake, local anesthetic, and no tourniquet (WALANT)
Hand and wrist surgeons have been using local anesthetic and no tourniquet for open procedures for some years now. Carpal tunnel decompressions are routinely done in this manner, and even Dupuytren’s fasciectomies are more commonly being performed through WALANT. The technique for arthroscopic surgery was described in 2012[54] using a small gauge needle, lidocaine mixed with adrenaline and buffered with sodium bicarbonate, and a slow methodical infiltration system. Other than lidocaine hypersensitivity and poor peripheral circulation, there are no contraindications to this technique, although the patient must be compliant. The use of WALANT also allows active dynamic assessment throughout the procedure[55] which enables a thorough assessment of carpal instability. With no tourniquet, the patient is more confortable and without the need for general anesthesia, the cost efficiency is immense.
NanoScope™
Needle arthroscopy is in its infancy, but has been used in the knee and shoulder for medial meniscectomies, labral, and rotator cuff repairs.[56-58] The advantages of single-incision, near scarless surgery are innumerable as long as the right instrumentation is available. A design team from Vanderbilt has recently manufactured a miniature needle-sized instrument with its own “wrist”[59] allowing greater degrees of freedom when instrumenting using the NanoScope™. The small scope in itself has allowed the therapeutic debridement of the trapeziometacarpal joint and the metacarpophalangeal joints.
One of the current problems associated with using the NanoScope™ is the issue of working at a distance [Figure 5]. The instrumentation is still developing and at present, it only has a 0 degree scope with a monitor of relatively poor resolution, compared with the standard stack resolution.
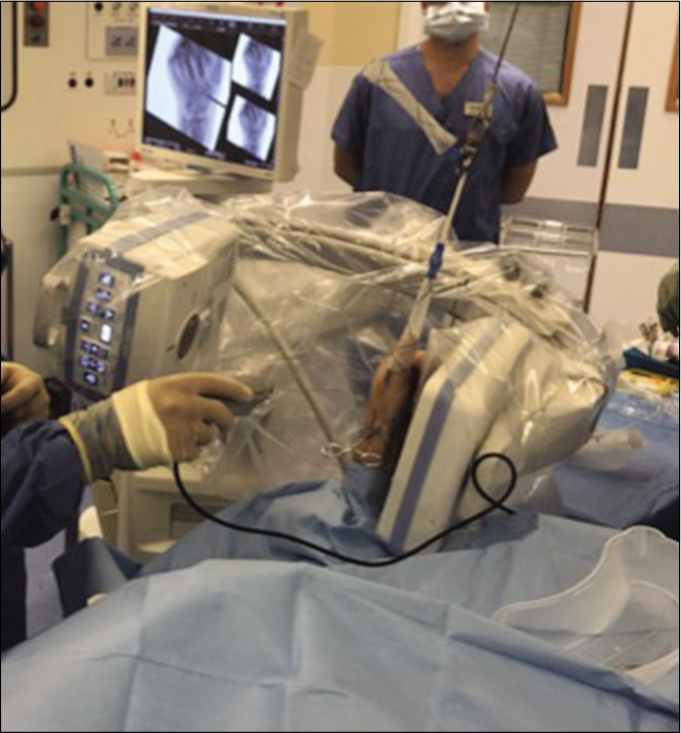
- NanoScope™ being used needs to be done standing and instrument size and working length necessitates surgeons to stand an arm’s length away from patient’s wrist.
There have been brilliant developments though with a “peekaboo” probe [Figure 6] that has become almost indispensible when using the NanoScope™. This type of innovation applied to other instruments, such as a drill, would certainly drive needle arthroscopy techniques forward at pace and allow assessment and treatment of smaller joints such as the trapeziometacarpal joint.

- “Peekaboo” probe used in NanoScope™ procedures, which allows insertion of instrument and probing of a surface in a different plane.
CONCLUSION
Wrist arthroscopy has come a long way over the past decade and has certainly caught up with its more proximal cubital neighbor. What was once known as a purely diagnostic intervention has now firmly landed in the therapeutic corner. Techniques of fracture fixation, bone grafting and ligament reconstruction, have all flourished with the advent of dry arthroscopy. With WALANT now well and truly underway, persistent chronic wrist pain can now readily be diagnosed and treated with minimal risks.
One of the major issues that continue to thwart us is the inability to see around corners. We are also limited by the working length of instruments, which need to be very thin and are, therefore, prone to breakage. New engineering concepts with “peekaboo” instruments and flexible instruments may see that problem slowly disappears. Certainly, the prospect of minimizing post-operative stiffness provides huge impetus to continue to drive arthroscopic advances forward in the wrist.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- (1882-1956) the first knee surgeon to use diagnostic arthroscopy. Arthroscopy. 2003;19:771-6.
- [CrossRef] [Google Scholar]
- Evaluation of chronic wrist pain by arthrography, arthroscopy, and arthrotomy. J Hand Surg Am. 1993;18:815-22.
- [CrossRef] [Google Scholar]
- Arthroscopic wrist anatomy and setup. Wrist and Elbow Arthroscopy 2015:1-28.
- [CrossRef] [Google Scholar]
- Intracarpal soft-tissue lesions associated with an intra-articular fracture of the distal end of the radius. J Bone Joint Surg Am. 1996;78:357-65.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of wrist arthroscopy: A multicenter study based on 10,107 arthroscopies. J Wrist Surg. 2016;5:320-6.
- [CrossRef] [PubMed] [Google Scholar]
- New advances in wrist arthroscopy. Arthroscopy. 2008;24:355-67.
- [CrossRef] [PubMed] [Google Scholar]
- Wrist arthroscopy through a volar radial portal. Arthroscopy. 2002;18:624-30.
- [CrossRef] [PubMed] [Google Scholar]
- Volar central portal in wrist arthroscopy. J Wrist Surg. 2016;5:80-90.
- [CrossRef] [PubMed] [Google Scholar]
- The value of wrist arthroscopy. An evaluation of 129 cases. J Hand Surg Br. 1996;21:210-2.
- [CrossRef] [Google Scholar]
- Management of chronic peripheral tears of the triangular fibrocartilage complex. J Hand Surg. 1991;16:340-6.
- [CrossRef] [Google Scholar]
- Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19:511-6.
- [CrossRef] [PubMed] [Google Scholar]
- The suction test: A novel technique to identify and verify successful repair of peripheral triangular fibrocartilage complex tears. J Wrist Surg. 2017;6:334-35.
- [CrossRef] [PubMed] [Google Scholar]
- New trends in arthroscopic management of Type 1-B TFCC injuries with DRUJ instability. J Hand Surg Eur Vol. 2009;34:582-91.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical comparison of arthroscopic versus open repair of triangular fibrocartilage complex tears. J Hand Surg. 2008;33:675-82.
- [CrossRef] [PubMed] [Google Scholar]
- Patient-reported outcomes following arthroscopic triangular fibrocartilage complex repair. J Wrist Surg. 2020;9:58-62.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of an arthroscopic orthopaedic procedure on a professional tennis player's career. Cureus. 2019;11:e5654.
- [CrossRef] [Google Scholar]
- Outcomes after wrist arthroscopy for the treatment of scapholunate predynamic instability in the young active patient. J Hand Surg Glob Online. 2019;1:174-7.
- [CrossRef] [Google Scholar]
- Arthroscopic dorsal capsuloligamentous repair in chronic scapholunate ligament tears. Hand Clin. 2011;27:563-72.
- [CrossRef] [PubMed] [Google Scholar]
- Wrist arthroscopy in degenerative conditions of the wrist. Arthroscopy 2016:913-30.
- [CrossRef] [Google Scholar]
- An articular-based approach to Kienbock avascular necrosis of the lunate. Tech Hand Up Extrem Surg. 2011;15:41-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of wrist arthroscopy in Kienbock disease. Hand Clin. 2017;33:727-34.
- [CrossRef] [PubMed] [Google Scholar]
- Wrist arthroscopy: Indications and results. Arthroscopy. 1992;8:198-203.
- [CrossRef] [Google Scholar]
- Foveal TFCC tear classification and treatment. Hand Clin. 2011;27:263-72.
- [CrossRef] [PubMed] [Google Scholar]
- All-arthroscopic triangular fibrocartilage complex ligamentoplasty for chronic DRUJ instability. Tech Hand Up Extrem Surg. 2019;23:44-51.
- [CrossRef] [PubMed] [Google Scholar]
- Ulnar impaction syndrome: Ulnar shortening vs. arthroscopic wafer procedure. J Wrist Surg. 2014;3:98-100.
- [CrossRef] [PubMed] [Google Scholar]
- Role of wrist arthroscopy in scapholunate dissociation. Orthop Traumatol Surg Res. 2020;106:S89-99.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes for arthroscopic thermal treatment for scapholunate ligament injuries. J Wrist Surg. 2020;9:22-8.
- [CrossRef] [PubMed] [Google Scholar]
- Electrothermal collagen shrinkage. J Hand Surg. 2012;37:2165-7.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic scapholunate ligament reconstruction, volar and dorsal reconstruction. Hand Clin. 2017;33:687-707.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic ligamentoplasty of the dorsal and volar portions of the scapholunate ligament. J Hand Surg Am. 2013;38:2466-77.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic resection of dorsal wrist ganglia and treatment of recurrences. J Hand Surg Br. 2000;25:38-40.
- [CrossRef] [PubMed] [Google Scholar]
- Artrhoscopic resection of volar wrist ganglion: Surgical technique and case series. Rev Bras Ortop (Sao Paulo). 2019;54:721-30.
- [CrossRef] [PubMed] [Google Scholar]
- Dry arthroscopic excision of dorsal wrist ganglion. Arthrosc Tech. 2017;6:e207-11.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic wrist arthrolysis after wrist fracture. Arthroscopy. 2007;23:255-60.
- [CrossRef] [PubMed] [Google Scholar]
- A revolutionary use of wrist arthroscopy in paediatric arthrogryposis? A case report and review of the literature. MOJ Orthop Rheumatol. 2019;11:204-6.
- [Google Scholar]
- The learning curve and pitfalls of arthroscopic four-corner arthrodesis. J Wrist Surg. 2019;8:202-8.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic proximal row carpectomy using the volar central portal. Arthrosc Tech. 2017;6:e1427-30.
- [CrossRef] [PubMed] [Google Scholar]
- Why do we use arthroscopy for distal radius fractures? Eur J Orthop Surg Traumatol. 2018;28:1505-14.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopic treatment of translunate perilunate injuries, not dislocated (PLIND) J Wrist Surg. 2019;8:143-6.
- [CrossRef] [PubMed] [Google Scholar]
- Scaphoid union: The role of wrist arthroscopy. Hand Clin. 2017;33:677-86.
- [CrossRef] [PubMed] [Google Scholar]
- Dry arthroscopy of the wrist: Surgical technique. J Hand Surg Am. 2007;32:119-23.
- [CrossRef] [PubMed] [Google Scholar]
- Arthroscopically assisted bone grafting reduces union time of scaphoid nonunions compared to percutaneous screw fixation alone. J Wrist Surg. 2020;9:13-8.
- [CrossRef] [PubMed] [Google Scholar]
- Wide-awake wrist arthroscopy and open TFCC repair. J Wrist Surg. 2012;1:55-60.
- [CrossRef] [PubMed] [Google Scholar]
- Wide-awake wrist and small joints arthroscopy of the hand. Hand Clin. 2019;35:85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Single-incision rotator cuff repair with a needle arthroscope. Arthrosc Tech. 2020;9:e419-23.
- [CrossRef] [PubMed] [Google Scholar]
- Incisionless partial medial meniscectomy. Arthrosc Tech. 2020;9:e375-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoscopic single-incision anterior labrum repair. Arthrosc Tech. 2020;9:e297-301.
- [CrossRef] [PubMed] [Google Scholar]
- Design, fabrication, and testing of a needle-sized wrist for surgical instruments. J Med Device. 2017;11:0145011-9.
- [CrossRef] [PubMed] [Google Scholar]






