Translate this page into:
The Posterolateral Corner: Explanations and Outcomes

*Corresponding author: Robert F. LaPrade, M.D., Ph.D., Complex Knee and Sports Medicine, Twin Cities Orthopedics, 4010 W 65th St, Edina, MN 55423, United States. laprademdphd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: LaPrade RF, Floyd ER, Carlson GB, Moatshe G, Chahla J, Monson J. The posterolateral corner: Explanations and outcomes. J Arthrosc Surg Sports Med 2021;2(2):108-18.
Abstract
In this review, we examine the current understanding of posterolateral corner (PLC) injuries and treatment methods. We discuss the anatomy of the major structures of the PLC and the biomechanics of how these structures function together as a unit. The diagnosis using physical examination, radiographs, and magnetic resonance imaging is discussed. The development of an anatomic reconstruction technique is then described, along with the surgical technique and rehabilitation protocols. Anatomic-based reconstruction methods and a regimented rehabilitation protocol better restore the native biomechanics of the knee, and improve subjective and objective outcomes at follow-up.
Keywords
Posterolateral corner
Fibular collateral ligament
Lateral collateral ligament
Popliteofibular ligament
Popliteus tendon
Varus stress
INTRODUCTION
The posterolateral corner (PLC) has been called many things, but it has never been described as simple or straightforward. Historically, surgery on this “Dark Side of the Knee” resulted in poor outcomes due to a limited understanding of the normal anatomy and biomechanics, and a paucity of knowledge for how to optimally approach and treat injuries to this area. The primary static stabilizing constituents of the PLC are the fibular (lateral) collateral ligament (FCL), popliteus tendon (PLT), and the popliteofibular ligament (PFL). A Grade III PLC tear occurs with complete disruption to all three of these structures.[1] Combined injuries of the posterolateral structures and cruciate ligaments are frequent (80–87% of PLC injuries[2-4]), with combined anterior cruciate ligament (ACL) and PLC injuries being most often observed. By contrast, only 13–28% of Grade III PLC tears occur in isolation.[4,5] Concomitant neurological, vascular, meniscal, or cartilage injuries are not uncommon, especially if PLC tears occur in the context of a knee dislocation.
Surgery for PLC injuries is necessary for restoring varus and external rotational stability of the knee and is a prerequisite for successful outcomes in ACL or posterior cruciate ligament (PCL) reconstruction.[2,6-10] Unfortunately, the diagnosis of PLC injuries is sometimes missed (up to 70% at initial presentation[11]), especially in patients with injuries to multiple structures, and a variety of non-anatomic methods continue to be used in addressing PLC injuries.[4,12] In recent years, outcome studies have demonstrated that earlier management of Grade III PLC injuries results in improved objective outcome scores and radiographic parameters, that reconstruction of the primary restraints is superior to repair, and that anatomic reconstruction restores the native biomechanics of the knee and avoids overconstraint.[2,7,13-16]
STRUCTURES OF THE PLC, THEIR ANATOMIC LANDMARKS, AND BIOMECHANICAL FUNCTIONS
One of the main factors complicating healing of Grade III PLC injury is the bony anatomy of the lateral side of the knee. In comparison to the medial compartment, the convex shape of the lateral femoral condyle (LFC) and its articulation with the flat or convex lateral tibial plateau (LTP) makes the PLC inherently unstable.[17,18] As a functional unit, the structures of the PLC act primarily to restrain varus forces on the knee, along with coupled posterolateral rotatory instability (increases in both external and internal rotation have been observed after PLC sectioning).[5,6,17,19] The primary and secondary stabilizers of the PLC are described below.
FCL
The FCL attaches to the femur in a shallow depression 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle (LE). The center of the femoral attachment is 18.5 mm posterior to the femoral attachment of the popliteus tendon. The average length of the FCL is 70 mm, and it attaches approximately midway along the lateral fibular head, in a small depression within the biceps femoris bursa, 28.4 mm distal to the tip of the styloid [Figure 1 and Table 1].[6,19-21] Biomechanical studies have reported that a side-to-side difference (SSD) >2.2–2.7 mm in varus gapping may be indicative of an isolated FCL tear.[5,22] The FCL is the primary static restraint to varus gapping of the knee and also functions to resist external rotation, particularly during extension. It also acts as a secondary stabilizer against internal rotation and anterior tibial translation.[5,19]
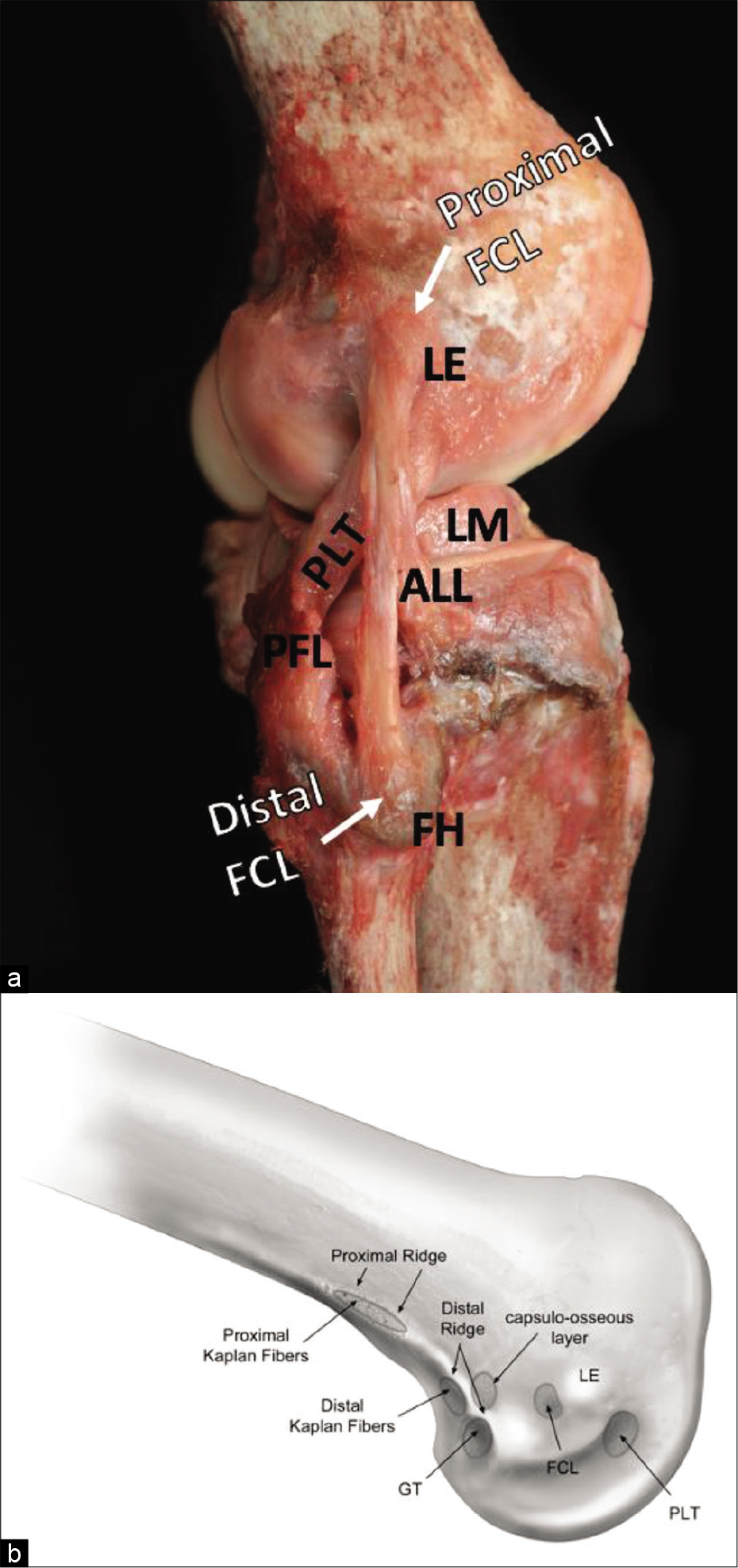
- Proximal and distal attachments of the fibular collateral ligament (FCL) and bony anatomy of the distal lateral femur. The proximal attachment of the FCL can be seen attaching in a shallow depression 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle (LE). (a) The anterolateral ligament (ALL) crosses obliquely from posterior to anterior relative to the FCL on its way to attach to the tibia, just inferior to the joint line. The popliteus tendon (PLT) attaches in the anterior fifth of the popliteus sulcus, 18.5 mm anterior to the proximal FCL attachment. The popliteofibular ligament can be seen in (a), arising from the musculotendinous junction of the PLT and attaching to the downslope of the styloid process of the fibular head. The lateral meniscus can also be seen. (b) Bony anatomy on the distal lateral femur, including the PLT sulcus, LE, FCL depression, and gastrocnemius tubercle.
| Pearls | Pitfalls |
|---|---|
| Perform posterolateral approach first to visualize the anatomy before fluid extravasation | Scarring and an avulsed BF tendon can make the location of the common peroneal nerve unpredictable. Go slow with the neurolysis |
| Incise the biceps bursa in order to identify the FCL fibular attachment, 28 mm distal to fibular styloid and 8 mm posterior to anterior border of fibula | Reaming the fibular head tunnel too proximally risks fracture or blowout |
| Tag any remnant FCL to assist in identifying femoral FCL origin | Validate that femoral PLT and FCL pins are 18.5 mm apart prior to reaming otherwise risk non anatomic placement or tunnel convergence |
| When drilling the tibial tunnel, place an obturator inside the fibular head tunnel to serve as a guide for the trajectory of the tibia PLC tunnel | If the PLC tibial guide pin is too lateral, it can enter the anterior compartment musculature or the PTFJ, particularly if the patient is obese |
| Tibial tunnel should exit 1 cm medial and 1 cm proximal to posteromedial exit to fibular tunnel | Make the femoral IT band incision anteriorly to prevent soft tissue from being difficult to retract during femoral tunnel prep |
| Use a large Chandler retractor with a finger behind it to protect the neurovascular bundle when drilling PLC tibial tunnel | Ensure both grafts passed under the ITB and the PLT is deep to the FCL graft |
| If having difficulty finding FCL femoral origin, make a small arthrotomy and identify PLT femoral origin, then measure 18.5 mm posterior | Aim femoral tunnels 35–40° proximal and posterior to avoid tunnel convergence with an ACL femoral tunnel |
| If needed, repair BF tendon in full extension after PLC reconstruction to avoid convergence between suture anchors and fibular tunnel | Remove reamers by hand to avoid damage to neurovascular structures |
PLT
The PLT originates on the anterior fifth of the popliteal sulcus and inserts in a broad aponeurotic attachment distal to the champagne-glass drop-off on the posteromedial tibial cortex. In extension, the tendon rests just above the sulcus and slips down to engage within the sulcus past 112° of knee flexion.[19] The average length of the PLT is 54.9 mm from its attachment on the sulcus to the musculotendinous junction of the popliteus, passing deep to the FCL.[23] The tendon courses posterodistally and passes through the popliteal hiatus, where the tendon anchors to the lateral meniscus with three popliteomeniscal fascicles.[19] The PLT functions as a ligament in the knee, providing a primary static constraint against external rotation of the tibia relative to the femur.[24] The tendon has a secondary role in stabilizing against internal rotation, varus gapping, and anterior tibial translation.[6,19]
PFL
The PFL consists of two divisions that originate at the musculotendinous junction of the popliteus muscle and insert distally and medially on the downsloping portion of the fibular styloid process. The larger posterior division inserts 1.6 mm distal to the styloid process tip; the smaller anterior division inserts more distally, 2.8 mm distal to the styloid tip [Figures 2 and 3].[17,19] The PFL acts as a primary static restraint against external rotation and as a secondary stabilizer against varus rotation. The effects of PFL deficiency are especially pronounced when other concomitant PLC injuries are present.[19] While often neglected, improved stability has been reported in Grade III PLC injuries when the PFL is reconstructed simultaneously with the FCL and PLT.[13,25]
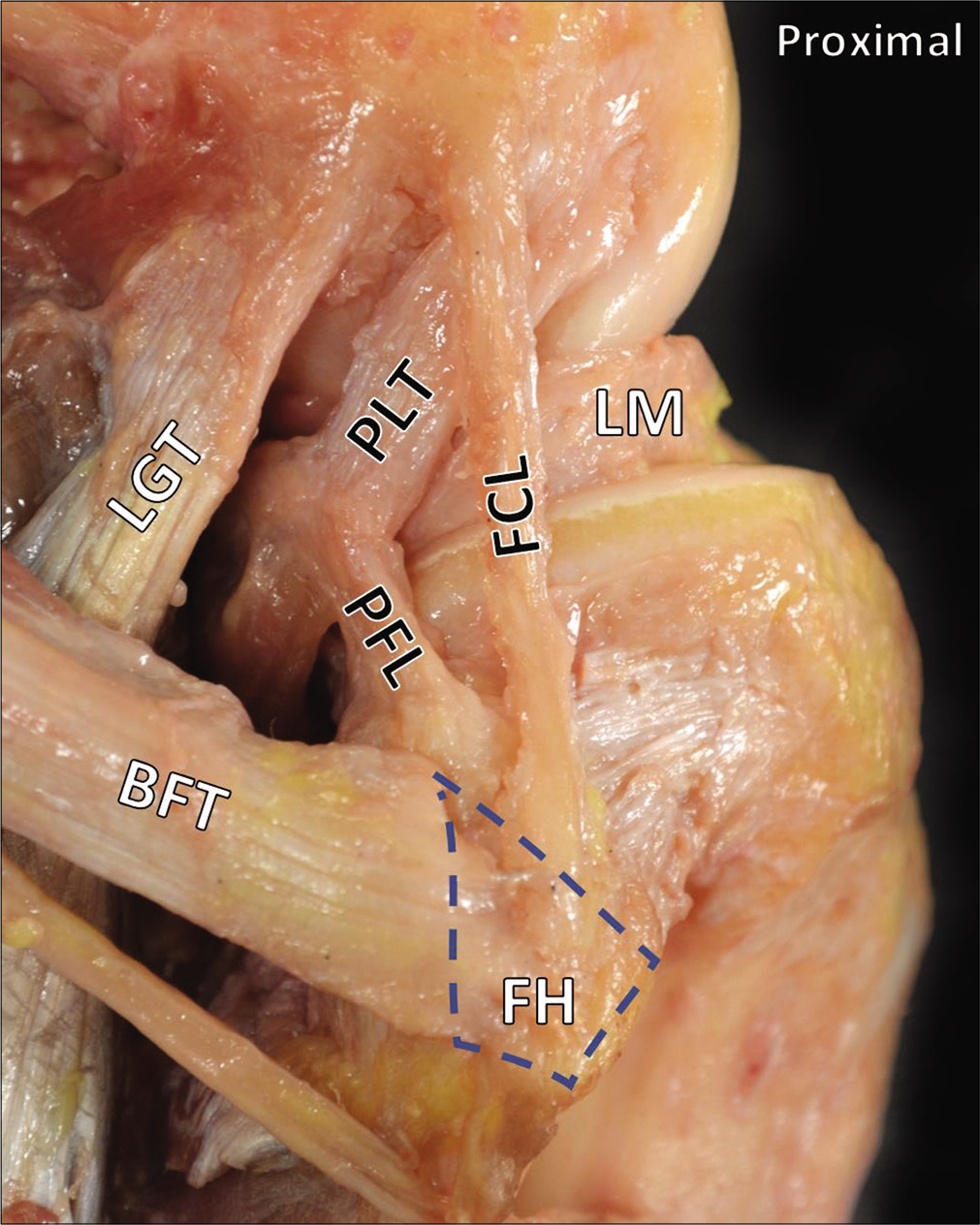
- The posterolateral corner. The three primary stabilizers of the posterolateral corner are the fibular collateral ligament (FCL), the popliteus tendon, and the popliteofibular ligament. Also pictured are the lateral gastrocnemius tendon and the lateral meniscus. The long head of the biceps femoris tendon attaches on the lateral aspect of the fibular head. Normally, the anterior arm would obscure the distal insertion of the FCL. In this image, the anterior arm has been removed to visualize the distal FCL. Normally, this distal attachment of the FCL is located in a bursa between the anterior and direct arms of the biceps femoris tendon.
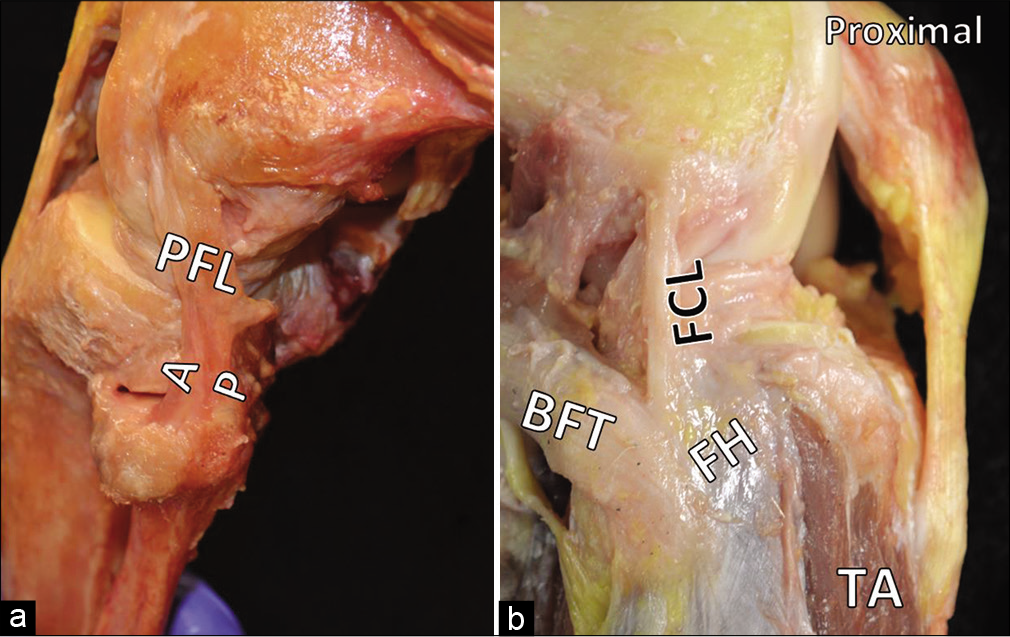
- Additional features of the posterolateral corner (PLC). In Part (a), the anterior (A) and posterior (P) divisions of the popliteofibular ligament are evident. Part (b) shows the anterior arm of the biceps femoris tendon obscuring the insertion of the fibular collateral ligament on the lateral fibular head. The proximal tibialis anterior is also visible and makes plain the danger to anterior compartment musculature if the tibial tunnel is malpositioned during PLC reconstruction.
SECONDARY STABILIZERS OF THE PLC
Several other structures act to augment the restraints on knee motion imposed by the three primary PLC structures, the FCL, PLT, and PFL. These include the fabellofibular ligament, which has been observed both with and without the presence of a bony fabella, though it is reported to be more substantial in subjects with a fully ossified fabella.[6,19] The distal iliotibial band (ITB) also plays a role in posterolateral stability, connecting the iliac crest of the pelvis to the patella, Gerdy’s tubercle, and the PLC. The distal ITB gives rise to the deep capsulo-osseous layer which has attachments to the lateral head of the gastrocnemius muscle, the biceps femoris, and the tibia posterior to Gerdy’s tubercle. The lateral gastrocnemius tendon attaches to the bony or cartilaginous fabella before continuing superiorly to insert on a supracondylar process on the posterior femur, 13.8 mm posterior to the proximal FCL and 28.4 mm from the PLT attachment, just superior to the joint capsule.[19] The proximal tibiofibular joint (PTFJ) ligaments also contribute to the stability of the PLC, connecting the medial proximal fibular head to the lateral tibia.[26] Anatomic PLC reconstruction techniques restore this articulation indirectly. In addition, isometric reconstruction techniques exist to restore the PTFJ if it is injured in isolation and symptoms prove resistant to conservative management.[27] The meniscotibial ligament connects the lateral meniscus to the tibia at its posterior aspect, providing additional stability to the posterolateral joint capsule.[5,19,28] The anterolateral ligament (ALL), a thickening within the “mid-third lateral capsular ligament,” has an oblique course superficial to the FCL and serves as a restraint against internal tibial rotation at 30° of flexion.[6,29,30] While not technically a part of the PLC, the ALL provides an important contribution to rotatory stability of the lateral side of the knee. Finally, the biceps femoris tendon (BFT) consists of two divisions:
The long head of the BFT divides into a direct and anterior arm and several fascial components. The direct arm inserts lateral to the fibular styloid; the anterior arm separates 1 cm proximal to the fibular head and inserts lateral to the fibular FCL attachment. The biceps bursa fills the space between these arms and encloses the distal FCL attachment, forming a convenient surgical landmark.[6,19,20]
The short head of the BFT also divides into an anterior arm (attaches 1 cm posterior to Gerdy’s tubercle) and a thicker direct arm, which inserts between the styloid and the FCL insertion. The short head of the BFT has numerous other connections to the capsulo-osseous ITB layer, posterolateral joint capsule, long head of the BFT, and distal femur.[5,19]
EVALUATION
History and physical examination
Isolated and non-contact PLC injuries are less common (although not absent) in the literature, and Grade III PLC tears are more often combined with other injuries.[5,31] Thus, patients typically remember an inciting event for their mechanism of injury. Mechanisms of injury include hyperextension with twisting, a blow to the anteromedial tibia in extension, landing with an outstretched extended leg, or high-energy trauma.[32] Patients often present with pain, swelling, and tenderness to palpation over the affected area.
A sound and thorough knowledge of relevant physical examination maneuvers is essential to differentiating PLC tears when large, multiligament injuries have been sustained. The patient may poorly localize pain after traumatic knee injuries or lose consciousness, and magnetic resonance imaging (MRI) may not clearly delineate PLC injuries when massive effusion or hematoma is present.[5,33,34] All knee examinations should be performed bilaterally and include varus stress testing at 20–30° and in extension, the dial test at 30° and 90°, reverse pivot shift, and heel height.
The external rotation recurvatum test was initially reported by Hughston (1980) as a way of grading the severity of PLC injuries by heel height [Figure 3].[35] Research later demonstrated that a positive external rotation recurvatum test with significant SSD in heel height is significant for a combined ACL and PLC injury.[36] More recently, a clinical study has reported an improved sensitivity and specificity of the heel height test over MRI for a minimum SSD of 2.5 cm in the heel height for detecting combined ACL and FCL tears.[37] As the distal thigh is stabilized with one hand and the foot lifted by the great toe with the other, a knee with a combined PLC and ACL injury will demonstrate an increased heel height as the tibia undergoes anterior subluxation and slips into external rotation.[32,35-37]
An increased SSD in varus gapping, with the femur stabilized and force applied through the foot, is presumed to indicate injury to the FCL (the primary varus stabilizer), and possibly other PLC structures. If gapping also occurs in extension, a combined ACL and PLC injury should be suspected. However, the varus stress test should be combined with other maneuvers and imaging to best determine the extent of the PLC injury.[38]
The dial test measures rotational stability; a test is positive when the clinician measures more than 10° of SSD in external rotation with the patient prone or supine and the knee flexed to 30°. At 90° of knee flexion, the influence of an intact PCL should decrease external rotation. If an increase in external rotation is seen at 90° of knee flexion instead, a combined PCL and PLC injury should be suspected.[5,32,33] A corollary is that disruption of posteromedial corner (PMC) structures has also been reported to increase external rotation. In this case, valgus stress gapping can be used to evaluate whether medial-sided injury is contributing to the observed increased external rotation.[39]
The reverse pivot shift test relies on the action of the ITB as a knee flexor in >30° of flexion. A supine patient’s knee is flexed to 90°, allowing the tibia to subluxate posteriorly when a PLC injury is present. As the knee is extended with external rotation and valgus force, the ITB sharply reduces the tibia around 30° when its major action changes to extension.[5,31,32]
Posterolateral rotational instability may present with a varus thrust gait causing significant pain during ambulation and ascending or descending stairs. This may only be able to be well assessed in patients with chronic injuries. Damage to the common peroneal nerve is a serious complication of these injuries and can result in a foot drop, most readily observable in the motor function of the extensor hallucis longus and dorsal sensation of the foot.
| Dial test | Increased external rotation at 90° | Decreased external rotation at 90° | Increased external rotation at 30° |
|---|---|---|---|
| Isolated posterolateral corner injuries | – | + | + |
| Cruciate ligament + posterolateral corner injury | + | – | + |
| Medial-sided injury | + | – | + |
Imaging
An initial screen for fractures or avulsions is performed with anteroposterior or lateral plain radiographs. Next, comparison of SSD of lateral opening with varus stress radiographs is used to assess FCL and PLC integrity [Figure 4]. LaPrade et al. (2008) demonstrated that > 4 mm of SSD in varus gapping is diagnostic of a complete posterolateral tear.[34] Varus gapping of as little as 2.2 mm has been reported to indicate a complete FCL tear.[22] It should be noted that stress radiographs sometimes can be difficult to perform in the acute phase.[40] Varus stress radiographs have been reported to increase the accuracy in the diagnosis of Grade III FCL tears over MRI alone.[38] MRI has been reported to have a sensitivity of anywhere from 58 to 100% for the diagnosis of PLC injury in the literature, but is highly recommended by these authors in assessment of isolated FCL and complete PLC injuries.[41,42] MRI sensitivity for PLC injuries has been reported to be superior in the acute setting than in the chronic setting.[11]
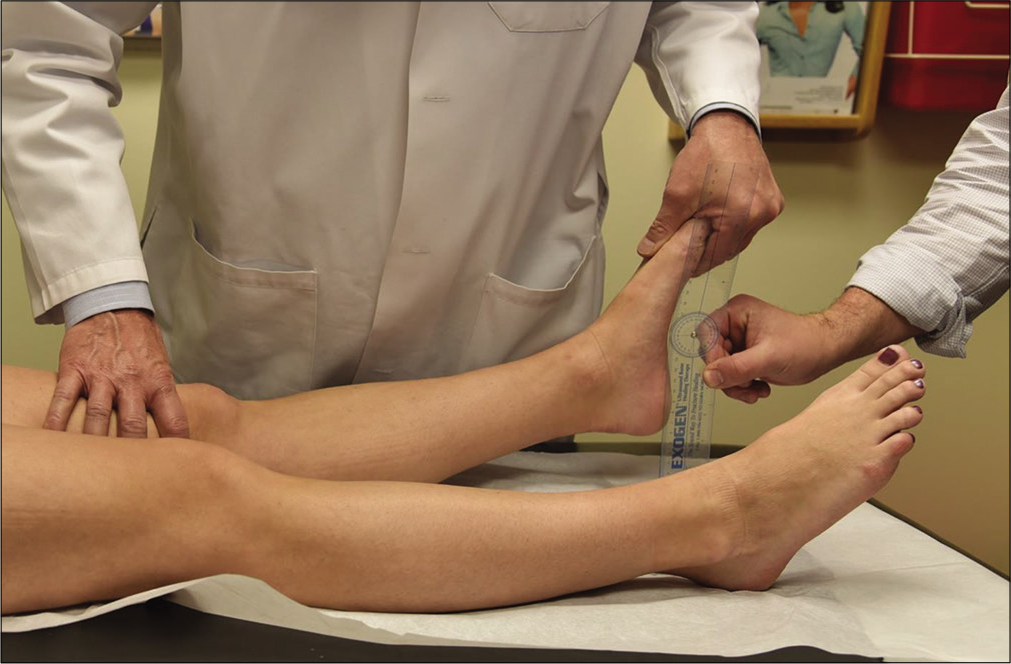
- The external rotation recurvatum test takes advantage of the increased heel height seen in patients with posterolateral corner (PLC) injuries. Historically used to grade PLC tear severity, the heel height test now is now understood to indicate the presence of a combined anterior cruciate ligament (ACL) and PLC injury. A side-to-side difference in heel height of 2.5 cm indicates a combined ACL/FCL tear. As the distal thigh is stabilized with one hand and the foot lifted by the great toe with the other, a knee with a combined PLC and ACL injury will demonstrate an increased heel height as the tibia undergoes anterior subluxation and slips into external rotation.
MRI assessment of PLC tears frequently reveals anteromedial bone bruises [Figure 5]. Geeslin and LaPrade (2010) reported on a cohort of 102 acute PLC injuries and found that 55% had anteromedial femoral condyle bruising. In addition, 11% of patients had anteromedial tibial plateau fractures and nearly 30% of patients with posteromedial tibial plateau bone bruising had concurrent ACL and PLC injuries.[43] Therefore, anteromedial bone bruising or medial tibial plateau fractures on MRI should be considered evidence of a PLC injury until proven otherwise.
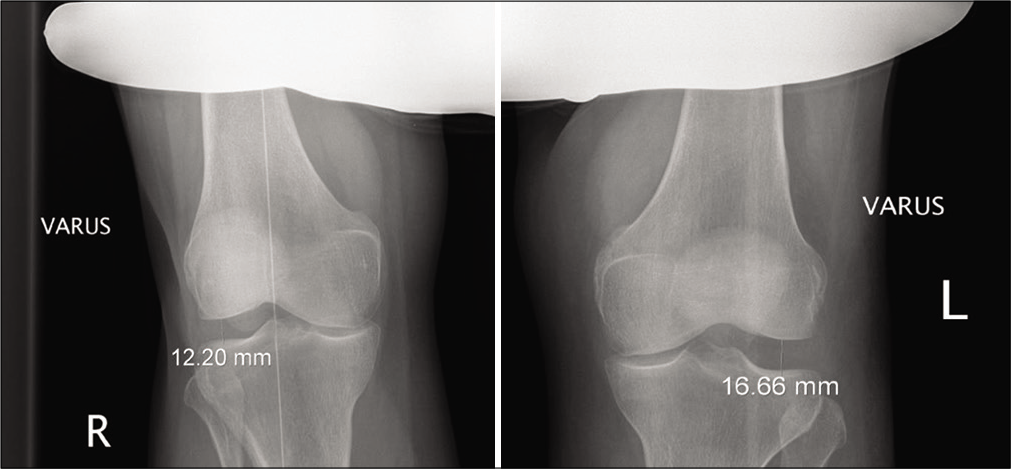
- Varus stress radiographs. Varus stress radiography is a validated tool for evaluating injuries of the posterolateral corner. A greater than 2.2 mm side-to-side difference in varus gapping between extremities indicates an isolated fibular collateral ligament tear. A difference in varus opening of >4 mm from one knee to the other indicates a complete posterolateral corner tear.
REPAIR VERSUS RECONSTRUCTION
The convex-on-convex articulation of the LFC and LTP creates an unstable environment around the PLC. For this reason, Grade III PLC injuries seldom heal with non-operative management, leading to persistent instability and reported progression to osteoarthritis.[1,2,5,11,17] Two prospective cohort studies evaluated repair versus reconstruction of PLC injuries and found a failure rate of close to 40% for attempted repairs of PLC injuries versus 6–9% failure for reconstructions.[2,44] Some authors have reported that acute repair may be superior to reconstruction at an extended interval after injury; however, not only are many injuries missed in the acute setting where repair is reported to be feasible,[11,45] methods of ligamentous reconstruction in the PLC have also varied widely.[13] Based on the results of a quantitative reanalysis of PLC anatomy performed by LaPrade et al. (2003), an anatomic-based surgical technique involving a reconstruction of the PLC of the PLC was developed [Figure 6]. Using two grafts and a fibular head tunnel, anatomic reconstruction restored the native anatomy and functional properties of the PLC, and was demonstrated with an in vitro biomechanical study to restore varus and external rotation to a functionally intact state.[17]
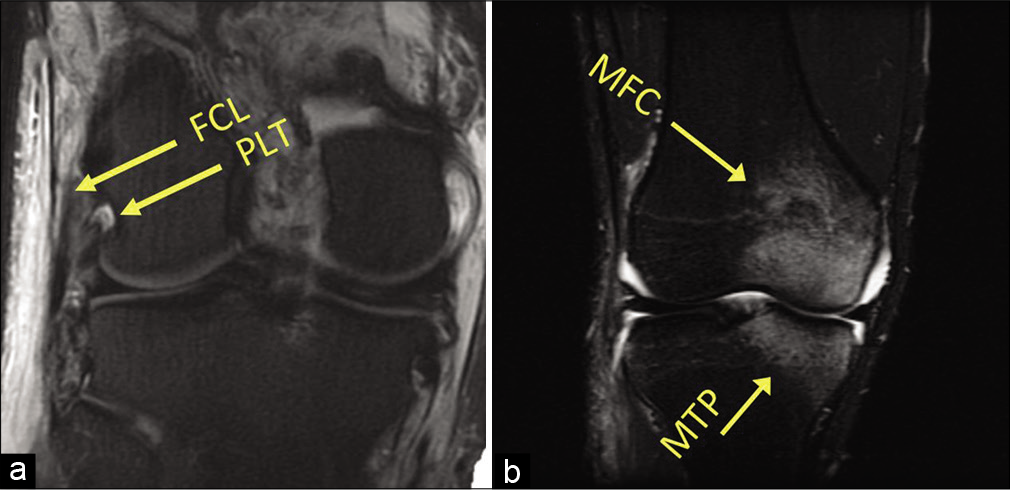
- Magnetic resonance imaging (MRI) assessment of posterolateral corner (PLC) injuries. MRI has a high sensitivity and specificity for the detection of Grade III (complete) PLC injuries in the acute setting. (a) A coronal T2-weighted MRI of a left knee. Attenuation of the fibular collateral ligament and avulsion of the popliteus tendon can be seen (arrows). In (b), a common radiographic sign of acute PLC injuries can be seen. Up to 55% of PLC tears demonstrate an anteromedial bone bruise, and this coronal T1-weighted fat-suppressed MRI image demonstrates increased signal in the medial femoral condyle and medial tibial plateau.
ANATOMIC POSTEROLATERAL KNEE RECONSTRUCTION TECHNIQUE
The authors’ method of anatomic reconstruction restores the FCL, PLT, and PFL using an Achilles’ tendon allograft split lengthwise to make two grafts. Each of the grafts is attached to a 9 × 20 mm bone plug and tubularized at the other end.[13] Other authors have since reported that the use of hamstring autografts can also be successful.[46] The surgical approach is made through a lateral hockey stick incision, with posterior dissection along the distal ITB and the long and short biceps femoris muscles. Care must be taken in this area; if the long biceps tendon is ruptured, scar tissue and malposition may obscure the common peroneal nerve. A neurolysis to decompress the nerve is performed and the nerve is gently retracted for its protection, but vessel loops are not used to avoid unnecessary traction. The distal attachment of the FCL to the lateral fibular head is identified by entering the biceps bursa between the anterior and direct arms of the long head of the biceps femoris. The integrity of the FCL can sometimes be assessed with a traction suture placed through its distal aspect; some remnant of the ligament may help locate its femoral attachment and to guide placement of the ITB splitting incision if any continuity remains between the distal and proximal remnants.[14] Retraction of an injured biceps femoris tendon or a longitudinal incision through the anterior arm of the long head of the biceps tendon 1 cm proximal to the fibular head may be necessary to clearly see the distal FCL attachment [Figure 2].[5] Using an elevator, the soleus musculature is elevated off of the posteromedial fibular head, allowing visualization of the musculotendinous junction of the PLT and the PFL. A guide pin is drilled from the FCL origin, 28 mm distal to the fibular styloid and 8 mm posterior to the anterior border of the fibula, through the fibular head in an anterolateral to posteromedial orientation to emerge just distal to the PFL attachment posteromedially. Then, the fibular tunnel can be reamed with a 7 mm reamer. A transtibial tunnel is then drilled with a guide pin, from the flat spot just distal and medial to Gerdy’s tubercle to the popliteus musculotendinous junction posteriorly.[5,13] This exit point is verified to be roughly 1 cm medial and 1 cm proximal to the posteromedial opening of the fibular tunnel, and a retractor is placed to protect the neurovascular bundle. This tunnel is then reamed with a 9 mm reamer.
Next, the ITB is incised in line with its fibers and the femoral attachment of the FCL is located 1.4 mm proximal and 3.1 mm posterior to the LE.[23] The remnant FCL can be used to identify the FCL sulcus, and any remnant FCL is then removed and an eyelet guide pin is drilled proximally and 35° anteriorly to avoid convergence with potential ACL reconstruction tunnels.[47] The PLT attachment is located in the popliteus sulcus, 18.5 mm anterior to the FCL attachment, and another eyelet guide pin placed parallel to the FCL pin [Figure 7]. Alternatively, the PLT pin can be placed first if there is difficulty with finding the FCL femoral origin by making a small arthrotomy and finding the PLT tendon on the anterior one-fifth of the popliteal sulcus. Before reaming the tunnels, verify the pins are distanced 18.5 mm apart. The pins are then overreamed with 9 mm reamers to a depth of 25 mm [Figure 8]. For both femoral tunnels, the eyelet guide pins are used to place passing stitches for insertion of grafts into the tunnels. The bone plugs of the PLT and FCL grafts are secured in their respective tunnels with 7 × 20 mm titanium interference screws. The PLT graft then is passed through the popliteal hiatus posteriorly, while the FCL graft is passed superficial to the PLT graft but deep to the ITB. The FCL graft is then passed through the fibular head tunnel, from lateral to medial, and secured at the lateral aspect with a 7 × 20 mm bioabsorbable interference screw with the knee in 20° of flexion, in neutral rotation, and with application of a gentle valgus force. The remainder of the graft emerging from the posteromedial aperture of the fibular tunnel functions as the PFL graft, and, along with the PLT graft, is passed through the tibial tunnel with the knee flexed at 60° and neutral rotation, and secured with another 9 × 20 mm bioabsorbable interference screw [Figure 9].
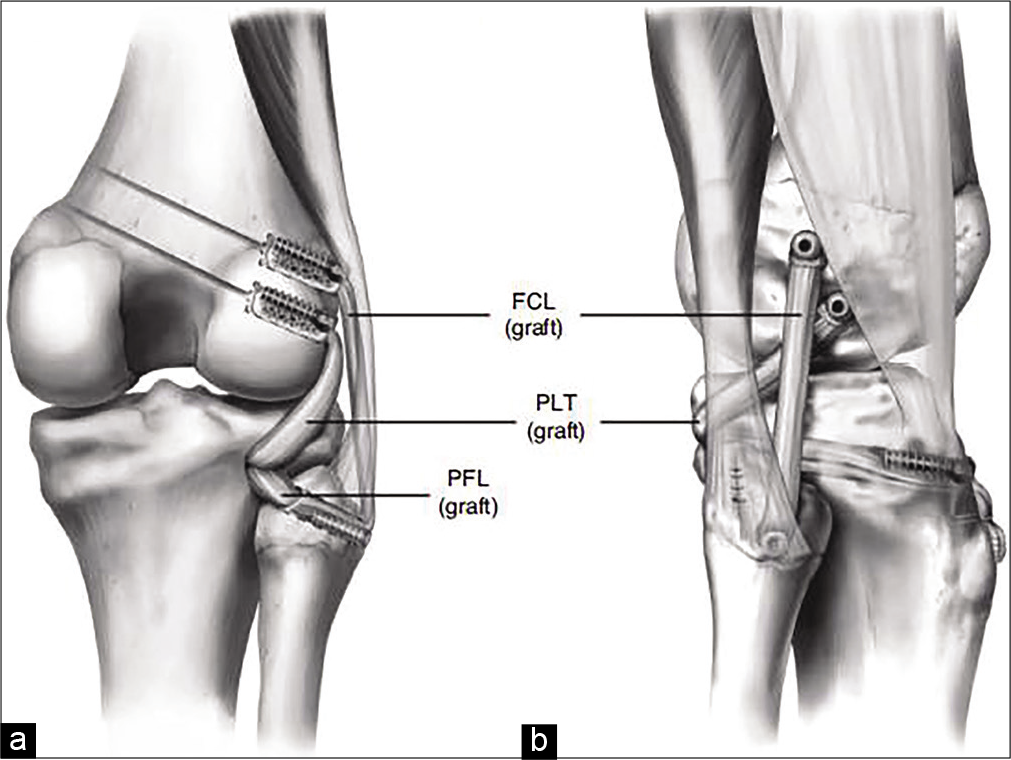
- (a and b) Anatomic reconstruction of the posterolateral corner (PLC). This image displays the final appearance of an anatomic PLC reconstruction procedure, which restores the native anatomy of the fibular collateral ligament, popliteofibular ligament, and popliteus tendon. The authors use a longitudinally split Achilles tendon allograft, with a fibular head and transtibial tunnel and interference screws for fixation. This image first appeared in LaPrade et al. AJSM:2004;32(6):1405-1413.
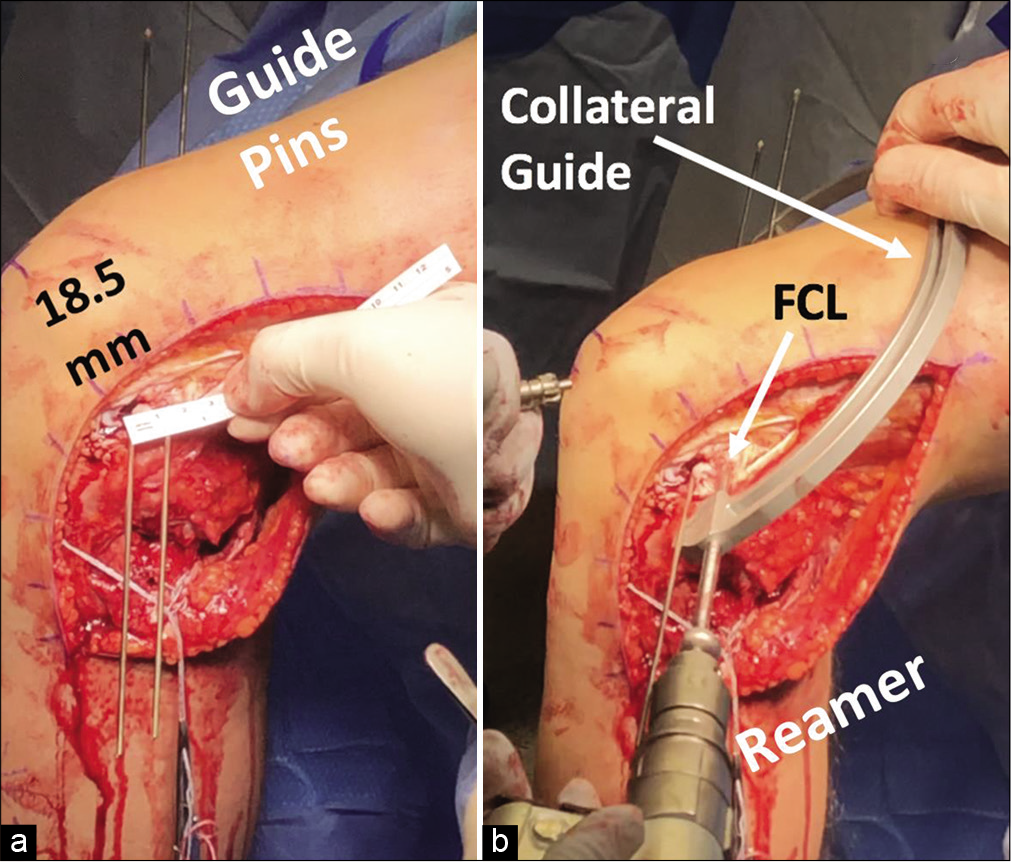
- (a and b) Creating tunnels for the fibular collateral ligament (FCL) and popliteus tendon (PLT) grafts in the femur. The anatomic attachment of the FCL on the lateral femoral condyle is 18.5 mm on average posterior to the PLT tendon attachment in the popliteal sulcus (ruler in a). During anatomic posterolateral corner reconstruction, a guide pin is first placed through the femoral attachment of the popliteus tendon. After the correct distance is verified, a guide pin is placed for the FCL femoral tunnel. When correct positioning of both pins is verified, a 9 mm reamer is then used to create FCL and PLT graft tunnels to a depth of 25 mm. A collateral ligament guide (b) is useful in overreaming the guide pins.
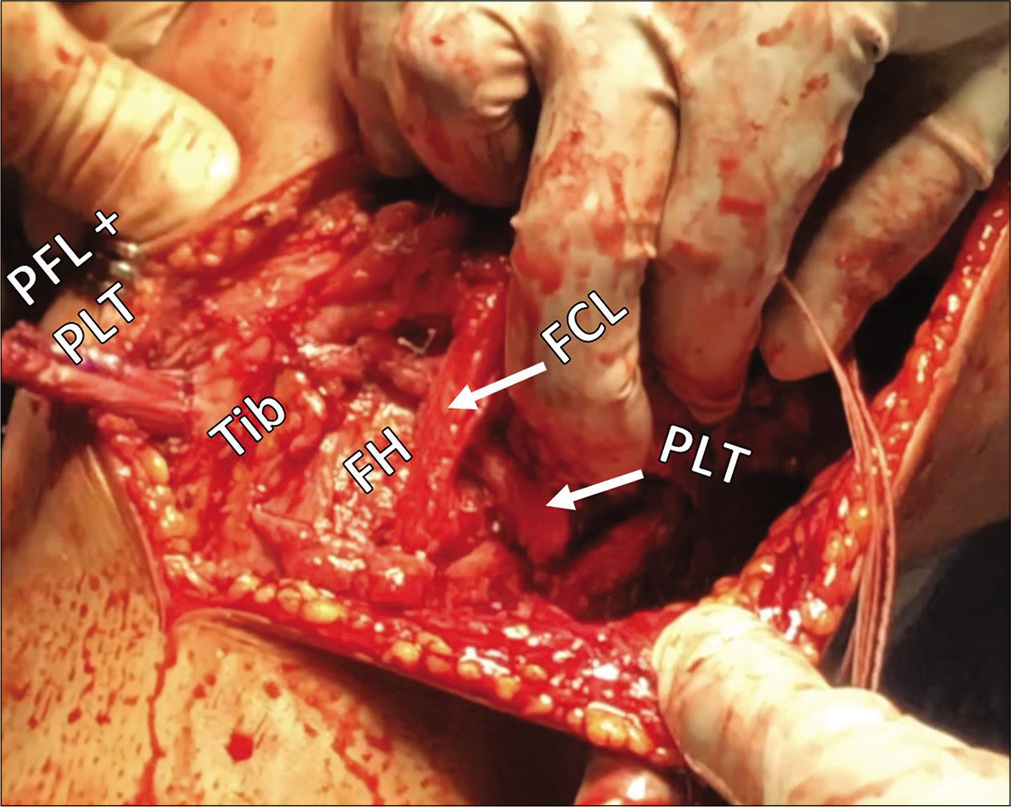
- Intraoperative view of anatomic posterolateral corner (PLC) reconstruction. Anatomic reconstruction of the PLC uses two grafts to restore three structures: The fibular collateral ligament (FCL), the popliteus tendon (PLT), and the popliteofibular ligament (PFL). The FCL graft passes anterolaterally to posteromedially through the fibular head tunnel and, along with the PLT, is passed posterior to anterior through a tibial (Tib) tunnel. After fixation in the fibular head, the remainder of the FCL graft serves as a PFL graft and stabilizes the proximal tibiofibular joint.
Timing of surgery
As previously mentioned, surgery in the acute setting is preferred. If surgery is delayed more than 6 weeks, outcomes have been reported to be comparable to chronic PLC tear reconstruction.[5,48] Chronic injuries can be more challenging to manage due to the accumulation of scar tissue and fibrosis; in these cases, any varus malalignment should be corrected before a PLC reconstruction to maximize the chances of a successful outcome. This prevents undue forces across the grafts which may lead to stretching out overtime.[5] Combined ACL and PLC injuries have also been reported to significantly increase the chances of ACL reconstruction failure if the PLC injury is not adequately addressed.[49]
REHABILITATION
Patients remain non-weight-bearing (NWB) for the first 6 weeks after surgery in a knee immobilizer that may be removed for range of motion activities and bathing. Routine vasopneumatic cryotherapy is initiated immediately after surgery to manage pain and swelling. Physical therapy begins on the 1st post-operative day with an emphasis on patient education, symptom management, knee motion, and quadriceps muscle activation. Knee flexion ROM is limited to 90° for the first 2 weeks then is progressed gradually until full knee flexion is recovered. Hyperextension is restricted to avoid excess strain on the PLC grafts. Patellofemoral joint mobilizations, peripatellar soft-tissue mobilizations, and frequent quadriceps contractions help minimize post-operative stiffness which is a critical for long-term joint health.[50-52] When biceps femoris repair is required, targeted hamstring muscle strengthening should be delayed for at least the first 8 weeks, per tissue and repair status, to minimize disruption of initial tendon healing at the attachment. Gentle stimulus to the tendon first occurs through active knee ROM. Intensity controlled hamstring isometrics may begin at 8 weeks, again per tissue quality, followed by graduated loading progressions to stimulate tendon healing.[53] We delay any isolated, resisted hamstring strengthening into knee flexion (curling) until 4 months post-operative with PLC reconstructions to avoid excessive posterior tibial translation.[54,55] The knee immobilizer provides joint support early on with NWB strengthening exercises when quadriceps force output is depressed and it may be removed once the patient no longer demonstrates a quadriceps lag with leg lifting. Exercises that induce knee varus, tibial external rotation or posterior translation, or knee hyperextension should be avoided in early rehab. Neuromuscular electrical stimulation (NMES) and blood flow restriction therapy are utilized to recover quadriceps muscle strength and minimize atrophy.[56-58] Patients may begin a gradual return to full weight-bearing at 6 weeks and discontinue crutch use when able to ambulate without a limp. They will also transition to a hinged knee brace at this time per quadriceps muscle control. Weight-bearing strength activities, balance training, and low impact cardiovascular fitness may commence once full weight-bearing is well tolerated. Squat depth is limited to 70° in the first 4 months.[59] Therapy programming should advance in structure and intensity to stimulate recovery of muscle endurance, hypertrophy, strength, and finally power.[60] Serial physical performance testing is useful to objectively measure patient progress with ROM, muscle strength, balance, and other functional and sporting tasks. Testing provides meaningful information, beyond strictly time from surgery, to guide decision-making regarding return to activities and sport.[5,13,61,62]
OUTCOMES
Geeslin et al.[63] and Moulton et al.[15] reported results of systematic reviews of acute and chronic PLC surgical management, respectively. In the acute setting, reconstruction of the PLC was associated with 81% success rate and 19% failure rate, as measured by a combination of post-operative Lysholm scores, International Knee Documentation Committee (IKDC) scores, and varus stress examination and radiography. This compared favorably with failure rates of 40% and 37% in attempts to directly repair Grade III PLC injuries.[44,64] In the chronic setting, surgical management of PLC injuries had a 90% success rate across 456 patients reviewed. Both of these studies included various techniques of PLC reconstruction.[15,63]
An early study by Yoon et al. (2006) compared an anatomic reconstruction, similar to the technique detailed by LaPrade, in 25 patients with a fibular sling technique in another 25 patients, and found significant improvements in Lysholm scores, along with varus opening and external rotational laxity at follow-up with the anatomic-based technique.[65] Outcomes of the chronic anatomic reconstruction technique detailed here, previously validated in a biomechanical study, were reported by LaPrade et al. (2010).[13,17] Reconstruction with the surgical technique and rehabilitation protocols detailed above resulted in an average Cincinnati score of 65.7, and significant improvement of IKDC scores for varus opening at 20°, external rotation at 30°, reverse pivot shift, and single-leg hop at an average of 4.3 years follow-up.[13] A prospective study of various combinations of acute repair, anatomic reconstruction, and hybrid techniques by Geeslin and LaPrade (2011) reported improvements in IKDC objective scores, varus gapping with stress radiographs, and significant improvements in IKDC and Cincinnati subjective outcomes with anatomic reconstruction.[14] Taken together, along with the results of studies by Stannard, Levy, and Black already discussed, the most current literature available on tears of the PLC suggest surgical reconstruction with anatomic-based techniques provides better outcomes at follow-up, with fewer complications than repair and non-anatomic reconstruction techniques.
CONCLUSION
PLC tears are potentially devastating injuries with a significant risk of long-term sequelae, arthrofibrosis, and progression to osteoarthritis if not properly addressed. The FCL, PLT, and PFL are the primary stabilizers of the PLC, serving as primary stabilizers against varus opening and external rotation and secondary stabilizers against anterior tibial translation. Concomitant cruciate ligament injuries are common, and in the acute setting, PLC tears are often missed. Over the past three decades, research has indicated to clinicians that reconstruction is superior to repair and that early instead of late management of these injuries allows for better long-term prognosis. Anatomic reconstruction techniques were developed with the help of biomechanical studies and validated in prospective outcomes studies as the most effective methods for restoring normal varus and rotational resistance in the knee and providing the best subjective outcomes and radiologic evidence of healing at follow-up.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Robert LaPrade: Consultant for Arthrex, Ossur, Smith and Nephew, and Linvatec Royalties: Arthrex, Ossur, and Smith and Nephew Research grants; Smith and Nephew and Ossur Editorial Boards: AOSSM, JEO, and KSSTA Committees: AOSSM, AANA, and ISAKOS
References
- Treatment or acute and chronic combined anterior cruciate ligament and posterolateral knee ligament injuries. Sports Med Arthrosc Rev. 1997;5:91-9.
- [CrossRef] [Google Scholar]
- Repair versus reconstruction in acute posterolateral instability of the knee. Sports Med Arthrosc Rev. 2015;23:22-6.
- [CrossRef] [PubMed] [Google Scholar]
- Novel approach for reconstruction of the posterolateral corner using a free tendon graft technique. Sports Med Arthrosc Rev. 2006;14:28-36.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23:1341-7.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral corner of the knee: Current concepts. Arch Bone Jt Surg. 2016;4:97-103.
- [Google Scholar]
- Anatomy and biomechanics of the posterolateral corner of the knee. J Knee Surg. 2005;18:137-45.
- [CrossRef] [PubMed] [Google Scholar]
- Improving outcomes for posterolateral knee injuries. J Orthop Res. 2014;32:485-91.
- [CrossRef] [PubMed] [Google Scholar]
- A biomechanical study of replacement of the posterior cruciate ligament with a graft, Part II: Forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1997;79:381-6.
- [CrossRef] [PubMed] [Google Scholar]
- A biomechanical study of replacement of the posterior cruciate ligament with a graft, Part 1. Isometry, pretension of the graft, and anterior-posterior laxity. J Bone Joint Surg Am. 1997;79:375-80.
- [CrossRef] [PubMed] [Google Scholar]
- Biomechanical analysis of a posterior cruciate ligament reconstruction, Deficiency of the posterolateral structures as a cause of graft failure. Am J Sports Med. 2000;28:32-9.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral corner injuries of the knee: A serious injury commonly missed. J Bone Joint Surg Br. 2011;93:194-7.
- [CrossRef] [PubMed] [Google Scholar]
- Management of combined anterior or posterior cruciate ligament and posterolateral corner injuries: A systematic review. Orthop Traumatol Surg Res. 2014;100(Suppl 8):S371-8.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92:16-22.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: A prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93:1672-83.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the outcomes of posterolateral corner knee injuries, Part 2: Surgical treatment of chronic injuries. Am J Sports Med. 2016;44:1616-23.
- [CrossRef] [PubMed] [Google Scholar]
- Fibular collateral ligament anatomical reconstructions: A prospective outcomes study. Am J Sports Med. 2010;38:2005-11.
- [CrossRef] [PubMed] [Google Scholar]
- An analysis of an anatomical posterolateral knee reconstruction: An in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32:1405-14.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of healing of grade III posterolateral corner injuries: An in vivo model. J Orthop Res. 2004;22:970-5.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and biomechanics of the lateral side of the knee and surgical implications. Sports Med Arthrosc Rev. 2015;23:2-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic fibular collateral ligament reconstruction. Arthrosc Tech. 2016;5:e309-14.
- [CrossRef] [PubMed] [Google Scholar]
- The fibular collateral ligament-biceps femoris bursa, An anatomic study. Am J Sports Med. 1997;25:439-43.
- [CrossRef] [PubMed] [Google Scholar]
- Fibular collateral ligament: Varus stress radiographic analysis using 3 different clinical techniques. Orthop J Sports Med. 2018;6:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- The posterolateral attachments of the knee: A qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31:854-60.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: The fifth ligament of the knee. Am J Sports Med. 2010;38:543-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38:1674-81.
- [CrossRef] [PubMed] [Google Scholar]
- The forgotten joint: Quantifying the anatomy of the proximal tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2018;26:1096-103.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic reconstruction of chronic symptomatic anterolateral proximal tibiofibular joint instability. Knee Surg Sports Traumatol Arthrosc. 2010;18:1452-5.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative and qualitative assessment of posterolateral meniscal anatomy: Defining the popliteal hiatus, popliteomeniscal fascicles, and the lateral meniscotibial ligament. Am J Sports Med. 2019;47:1797-803.
- [CrossRef] [PubMed] [Google Scholar]
- The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43:1606-15.
- [CrossRef] [PubMed] [Google Scholar]
- The magnetic resonance imaging appearance of individual structures of the posterolateral knee. A prospective study of normal knees and knees with surgically verified grade III injuries. Am J Sports Med. 2000;28:191-9.
- [CrossRef] [PubMed] [Google Scholar]
- Injuries to the posterolateral aspect of the knee, Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25:433-8.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral corner injury of the knee: Evaluation and management. J Am Acad Orthop Surg. 2008;16:506-18.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the reliability of the dial test for posterolateral rotatory instability: A cadaveric study using an isotonic rotation machine. Arthroscopy. 2008;24:593-8.
- [CrossRef] [PubMed] [Google Scholar]
- The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries, An in vitro biomechanical study. J Bone Joint Surg Am. 2008;90:2069-76.
- [CrossRef] [PubMed] [Google Scholar]
- The posterolateral drawer test and external rotational recurvatum test for posterolateral rotatory instability of the knee. Clin Orthop Rel Res. 1980;147:82-7.
- [CrossRef] [Google Scholar]
- The external rotation recurvatum test revisited: Reevaluation of the sagittal plane tibiofemoral relationship. Am J Sports Med. 2008;36:709-12.
- [CrossRef] [PubMed] [Google Scholar]
- The heel height test: A novel tool for the detection of combined anterior cruciate ligament and fibular collateral ligament tears. Arthroscopy. 2017;33:2177-81.
- [CrossRef] [PubMed] [Google Scholar]
- Increased accuracy of varus stress radiographs versus magnetic resonance imaging in diagnosing fibular collateral ligament grade III tears. Arthroscopy. 2018;34:2230-5.
- [CrossRef] [PubMed] [Google Scholar]
- Medial knee injury: Part 1, static function of the individual components of the main medial knee structures. Am J Sports Med. 2009;37:1762-70.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of multiligament knee injury: State of the art. J ISAKOS. 2017;2:152-61.
- [CrossRef] [Google Scholar]
- Correlation between magnetic resonance imaging and physical exam in assessment of injuries to posterolateral corner of the knee. Acta Ortop Bras. 2014;22:124-6.
- [CrossRef] [PubMed] [Google Scholar]
- Posterolateral complex knee injuries: Magnetic resonance imaging with surgical correlation. Acta Radiol. 2005;46:297-305.
- [CrossRef] [PubMed] [Google Scholar]
- Location of bone bruises and other osseous injuries associated with acute grade III isolated and combined posterolateral knee injuries. Am J Sports Med. 2010;38:2502-8.
- [CrossRef] [PubMed] [Google Scholar]
- Repair versus reconstruction of the fibular collateral ligament and posterolateral corner in the multiligament-injured knee. Am J Sports Med. 2010;38:804-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acute posterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1983;65:614-8.
- [CrossRef] [Google Scholar]
- A hamstring-based anatomic posterolateral knee reconstruction with autografts improves both radiographic instability and functional outcomes. Arthroscopy. 2019;35:1676-85.e1673.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple ligament reconstruction femoral tunnels: Intertunnel relationships and guidelines to avoid convergence. Am J Sports Med. 2017;45:563-9.
- [CrossRef] [PubMed] [Google Scholar]
- Combined posterior cruciate ligament injuries. Clin Sports Med. 1994;13:629-47.
- [CrossRef] [Google Scholar]
- Surgical management and treatment of the anterior cruciate ligament/posterolateral corner injured knee. Clin Sports Med. 2017;36:105-17.
- [CrossRef] [PubMed] [Google Scholar]
- Clinic-based patellar mobilization therapy for knee osteoarthritis: A randomized clinical trial. Ann Fam Med. 2018;16:521-9.
- [CrossRef] [PubMed] [Google Scholar]
- Minimum 10-year results after anterior cruciate ligament reconstruction: How the loss of normal knee motion compounds other factors related to the development of osteoarthritis after surgery. Am J Sports Med. 2009;37:471-80.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of normal knee motion after anterior cruciate ligament reconstruction is associated with radiographic arthritic changes after surgery. Am J Sports Med. 2012;40:108-13.
- [CrossRef] [PubMed] [Google Scholar]
- Tendon healing: An overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc. 2015;23:2097-105.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32:1144-9.
- [CrossRef] [PubMed] [Google Scholar]
- Rehabilitation after posterior cruciate ligament reconstruction: A review of the literature and theoretical support. Arch Orthop Trauma Surg. 2013;133:1687-95.
- [CrossRef] [PubMed] [Google Scholar]
- Blood flow restriction therapy after knee surgery: Indications, safety considerations, and postoperative protocol. Arthrosc Tech. 2018;7:e1037-43.
- [CrossRef] [PubMed] [Google Scholar]
- Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br J Sports Med. 2017;51:1003-11.
- [CrossRef] [PubMed] [Google Scholar]
- Quadriceps activation following knee injuries: A systematic review. J Athl Train. 2010;45:87-97.
- [CrossRef] [PubMed] [Google Scholar]
- Cruciate ligament loading during common knee rehabilitation exercises. Proc Inst Mech Eng H. 2012;226:670-80.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts in periodization of strength and conditioning for the sports physical therapist. Int J Sports Phys Ther. 2015;10:734-47.
- [Google Scholar]
- Current concepts in the recognition and treatment of posterolateral corner injuries of the knee. J Orthop Sports Phys Ther. 2010;40:502-16.
- [CrossRef] [PubMed] [Google Scholar]
- 2016 consensus statement on return to sport from the first world congress in sports physical therapy, bern. Br J Sports Med. 2016;50:853-64.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the outcomes of posterolateral corner knee injuries, Part 1: Surgical treatment of acute injuries. Am J Sports Med. 2016;44:1336-42.
- [CrossRef] [PubMed] [Google Scholar]
- The posterolateral corner of the knee: Repair versus reconstruction. Am J Sports Med. 2005;33:881-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic reconstructive surgery for posterolateral instability of the knee. Arthroscopy. 2006;22:159-65.
- [CrossRef] [PubMed] [Google Scholar]







