Translate this page into:
History of regenerative medicine in the field of orthopedics

*Corresponding author: Abhishek Vaish, Department of Orthopedics, Indraprastha Apollo Hospitals, New Delhi, India. drabhishekvaish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vaish A, Murrell W, Vaishya R. History of regenerative medicine in the field of orthopedics. J Arthrosc Surg Sport Med 2020;1(1):154-158.
Abstract
The demand and surge of regenerative medical treatments for various musculoskeletal disorders and injuries have increased exponentially in the recent past. We have reviewed the evolution of these treatments, from the past to the present times. This era has seen a paradigm shift from the replacement to regenerative methods of treatment for many orthopedic disorders. The regenerative medicine helps in restoring the natural tissue in the body at the diseased area. From the ancient methods of provoking tissue healing by noxious stimuli, now, many sophisticated and scientifically proven techniques of regeneration of tissues have come up and are being used globally. Cell therapies have been used as a treatment for a variety of musculoskeletal pathologies including osteoarthritis, cartilage defects, tendinopathies, delayed union and non-unions, non-union of fractures, and treatment of avascular necrosis of femoral head and other bones. Cellular therapies, with or without tissue engineering, seem to the future of regenerative medicine and these may make the replacement of a diseased joint or bone redundant in the near future.
Keywords
Stem cells
Regeneration
Orthopedics
Healing
Cartilage
BACKGROUND
The potential of living vital tissue to heal and repair itself has been known for ages. However, what was known was quickly lost in time due to a lack of insight and energy to pursue. Only recently in over the past four decades, the surge to develop techniques to restore the natural tissue in the body has risen.[1] This obviates the need for replacement of body parts till later in life. We hereby analyze the history and evolution of regenerative medicine mainly relevant to the field of orthopedics. Regenerative medicine along with biological methods has been recognized as the next-generation advances in the treatment of musculoskeletal conditions. There are various exciting available options at present which include autologous blood derivatives such as platelet- rich plasma (PRP), purified cytokines, and cell-based therapy.[2] According to recent literature cell therapy, among these appear to have the most potential for augmentation of tissue healing and regeneration. It is well known that due to their poor vascularity, musculoskeletal tissues such as cartilage, meniscus, and intra-articular ligaments do not heal. Seldom, even bone fails to heal after an injury or fracture. Hence, there is a need for the regeneration of these tissues which requires four key components: Cells, morphogenetic signals, scaffolds, and an appropriate mechanical environment.[3,4] The strategies of treatment to regenerate tissue could include stimulation of healing response, genetic alteration, cellular signaling changes, and exogenous augmentation with the help of scaffolds.[5] This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms. ©2020 Published by Scientific Scholar on behalf of Journal of Arthroscopic Surgery and Sports Medicine
EVOLUTION OF REGENERATIVE MEDICINE
Older past
Research and clinical application pertaining to regenerative medicine may be recent; however, its presence and importance were well known even in ancient times as they knew that when noxious stimuli are applied to injured tissue, it can induce healing. This fact has been traceable to 500 BC in Rome. Hot needle therapy was a common form of treatment for soldiers with joint dislocations.[6] In the 20th century, prolotherapy became quite common. It is a procedure where hyperosmolar substances were injected into damaged tissue to induce healing. Hippocrates identified that cartilage damage was associated with severe morbidity. Hence, multiple techniques were attempted to heal this damaged cartilage. Some such procedures were bloodletting, Roman baths, medicinal herbs consumption, and acupuncture.[7]Non-operative treatment was practiced even in the 1930s. A general surgeon from Philadelphia used sclerosing agents to treat a thumb injury and later the treatment of painful hypermobile joints with little success.[8]
Recent past
The regenerative medical treatment for the knee osteoarthritis (OA) and articular cartilage injuries of the joints has been tried in the last century. A timeline for various landmark therapies is shown in [Figure 1].
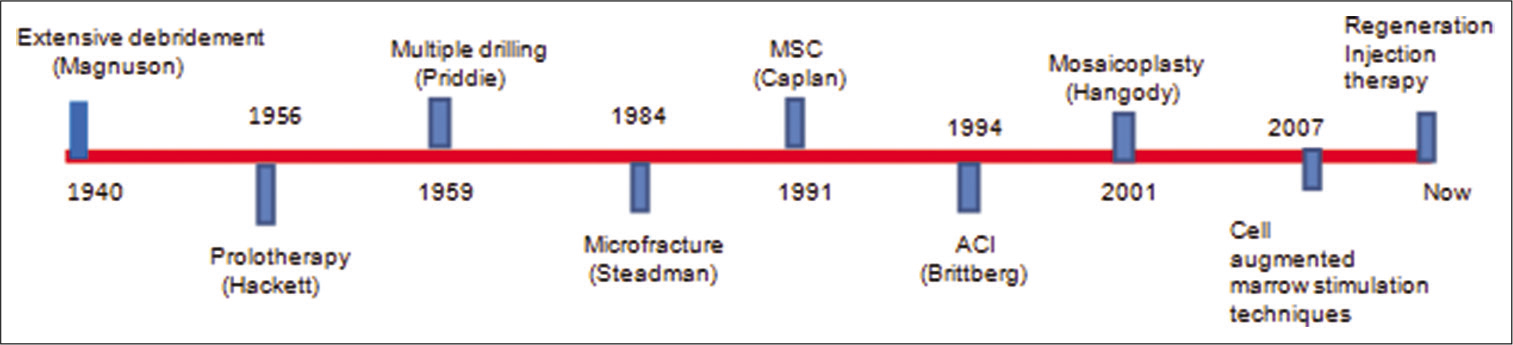
- Timeline of evolution of the treatment of symptomatic cartilage lesions.
In the 1940s, the treatment for OA was conservative until it was Magnuson who described extensive debridement and removal of loose degenerated particles of cartilage, synovium, and osteophytes from the knee, thus inducing a healing response.[9] This procedure was popular for many years until it was replaced with formal arthroplasty. Hackett et al. (1956) laid the foundation for prolotherapy.[10,11]According to his theory, peripheral joints which were painful were a result of axial instability and referred neural input with loss of muscular and ligamentous control. He used this novel technique for the treatment of arthritic joints. Pridie (1959) was working relentlessly in the United Kingdom. He expanded on the previous work of Magnuson and presented a technique of closely spaced multiple drilling of knee arthritic articular cartilage defects to promote a regenerative response. He could not show his clinical outcomes at long-term follow-up at this time.[12] However, it was Insall (1974) who performed this procedure for 60 patients and achieved excellent results in selected patients.[13]Microfracture (MFx) was initially discovered only to treat cartilage loss which was a full thickness in nature, unlike Pridie drilling was more for the treatment of arthritis.[14] The initial technique was described in 1984; however, the clinical results showing its usefulness in the long term were reported in 2003, by Steadman et al. much later with average 11-year follow-up.[15] Following these results, many researchers were interested in this technique which leads to various studies. It was concluded that the size and location of the lesion were important in the prognosis of the disease. The smaller lesions located on the femoral condyles and trochlea showed superior results with this method of treatment. However, larger and multifocal lesions and those located on patellar sites still did not show reproducible superior outcomes and thus presented a treatment dilemma. Mosaicplasty was a newer technique discovered and popularized by Hangody and Füles.[16] In 2004, he published his results in 831 patients. According to this study, he achieved excellent results for 92% (femoral), 87% (tibial), and 79% (patellar) lesions. It is a procedure where cartilage (osteochondral plug) is harvested from the healthy non-weight-bearing donor site and transported to the debrided and prepared recipient site. Only 3% of patients in his study showed mild donor site morbidity.
Autologous chondrocyte implantation (ACI) was another new technique which was developed by Brittberg et al.[17] This technique involved the culture of articular chondrocytes in a laboratory for 3–6 weeks. These cells were then reimplanted onto the prepared lesion site. There are four generations of this technique. In the first generation, ACI cells used to be injected below a periosteal patch so that they were contained. However, this went into disrepute due to its complications of patch dislodgement and hypertrophy. The second- and third- generation ACI used biodegradable polymers as delivery systems. The fourth-generation ACI technique is essentially gel based and specifically used culture-expanded bone marrow- derived cells that demonstrated excellent short-term safety and efficacy to autologous chondrocytes for focal cartilage lesions.[18]
Caplan also known as the father of mesenchymal stem cell coined the term ‘‘MSC”.[19] Later, marrow stimulation procedures such as MFx and/or drilling were combined with the application of various sources of stem cells such as concentrated bone marrow aspirate (BMAC), adipose- stromal vascular fraction (A-SVF), culture expanded-adipose derived stem cells, and peripheral blood stem cells. These were found to be successful in short- to mid-term follow-up. However, long-term follow-up with large sample sizes was still needed to alter the clinical practice.
Over the years, medical advances and quest to find the greatest advantages with minimally invasive procedures gave birth to the age of regenerative injection therapy (RIT). Linetsky[20] coined the term regenerative injection therapy or RIT. He expanded on the work already done by Gedney, Hackett, and Hem- wall and considered injecting various agents that induce a biological response, such as sodium bicarbonate/calcium gluconate, hyaluronic acid (HA),[21]dextrose, BMAC, PRP, nano or micronized fat, A-SVF, and culture-expanded MSCs both allogeneic and autologous from a multitude of sources.[22-25] The volume and quality of studies are on a steep rise and hence helping scientists all over the world to study the application of cells and if these are safe and efficacious. Demineralized bone matrix has been known for its osteoinductive properties and clinical applications for more than 5 decades.[26] Bone morphogenetic protein 2 and 7 were the active ingredients that were found to be clinically useful. These have already been cloned in the 1980s; however, they were approved for clinical use only in the 2000s. Despite having known the benefits of using stem cells for regeneration, progress in this field for clinical use has been slow. There is still a lack of efficient guidelines and ethical clearance to use these in clinical work. As the researchers have studied more about inflammation and its mediators in detail, PRP injections have come into extensive as a method to regenerate tissue.[27] Platelets carry inflammatory mediators which are critical for the healing process; hence, injecting a higher than the physiologic concentration of platelets could induce tissue regeneration. This was first tried as early as the 1970s. These platelets were similarly used in 1987 for an open heart surgery performed in italy. This became popular during the 1990s, for oral and maxillofacial surgeries. Many other fields were exploring this exciting technology of regeneration. Surgeons tried this to improve flap survival and bone healing. More recently, it is vastly being used for the treatment of sports injuries such as lateral epicondylitis, muscle ruptures, ACL tears, and heel pathologies.[28]
STEM CELLS
Any cell which has the ability to differentiate into specialized cell types and is capable of self-renewal is known as stem cells.[29] Based on their potential to differentiate and their potency, these can be classified into totipotent, pluripotent, multipotent, or unipotent cells [Figure 2]. Totipotent cells are the zygotes and early blastomeres and have the ability to form cells of all the types including the extra-embryonic tissues like a placenta. Pluripotent cells or embryonic stem cells are capable to form embryonic tissues from all the three primary germ layers. These are isolated from the inner cell mass of the blastocyst. In addition to the native pluripotent stem cells, it is now also possible to derive a pluripotent stem cell population from an adult somatic cell, experimentally. These cells are known as “induced” pluripotent stem cells (iPSCs).
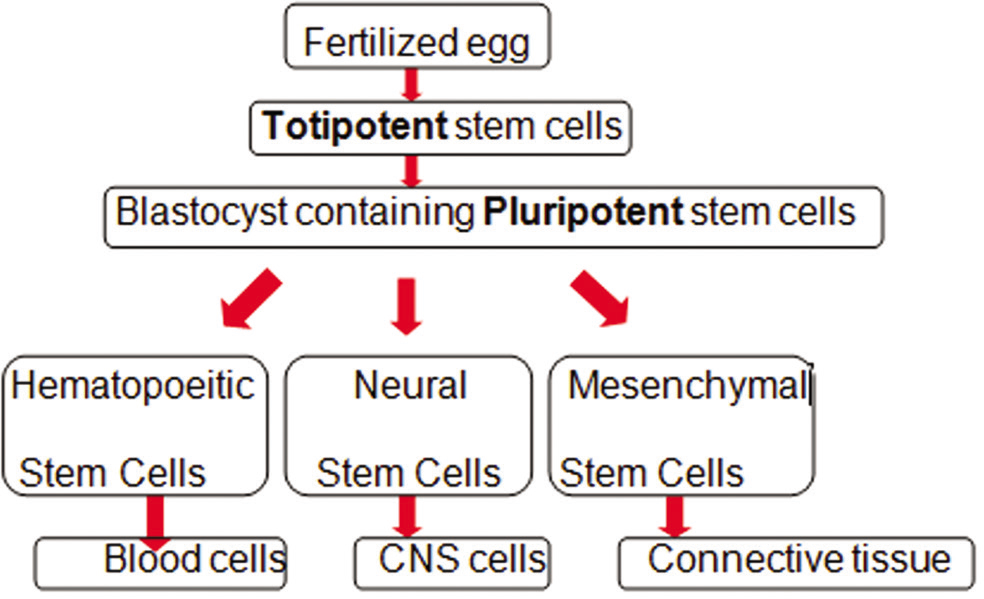
- Classification of stem cells.
The use of various types of stem cells perhaps can be credited to Weissman[30] who did the first bone marrow transplant on a patient with leukemia in 1956. Since then, various scientists have identified and used different types of stem cells for various indications [Figure 3] and have now become the basis of various regenerative medical treatments.
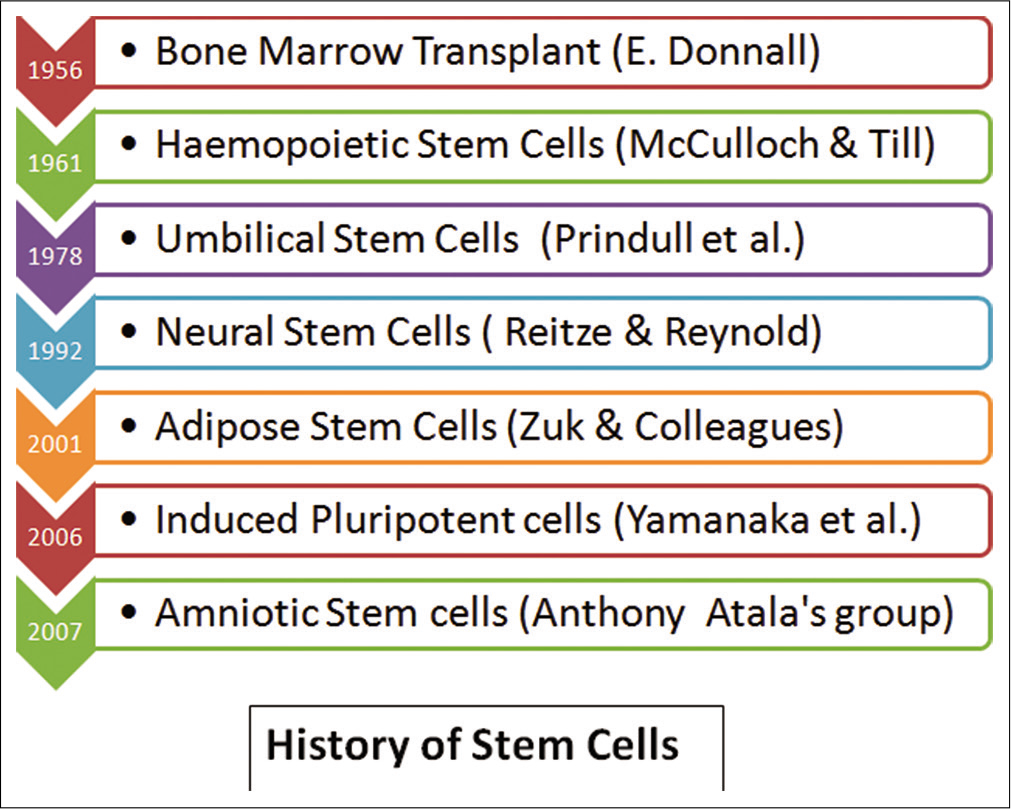
- History of stem cells.
ARTICULAR CARTILAGE
Cartilage was recognized as an important vital tissue, way back in the 4th century BC by Aristotle.[31] Hunter (1973) realized that if the cartilage is damaged, it was difficult to repair and restore.[32] Due to this belief and dictum, the researchers perhaps lost interest in trying to restore it, for almost 2 centuries.[33] It was only in the late half of the 19th century, interest in the field of regeneration started. Now, many techniques have evolved over the past years for cartilage repair. The journey of regeneration began with drilling and MFx and then moved toward autologous osteochondral transplantation, allografts, and ACI. Many two-staged procedures have evolved and shifting to single- stage arthroscopic techniques of cartilage transplantation. There has been a tremendous interest in the techniques of articular cartilage repair in the recent past and this field seems to be one of the most promising fields of orthopedics, which has seen a marked upsurge in the publications related to this field.[34,35]
TISSUE ENGINEERING
Tissue engineering techniques seem to have the promise of future tissue and cartilage regeneration. These techniques employ a variety of cell sources (e.g., autologous, allogeneic, xenogeneic, and stem cells).[36] We still do not know the “best” or a “gold standard” cell source. However, the stem cells seem most feasible and effective solution due to their easy availability and lack of donor site morbidity. Tissue- engineered products such as cell-based, cell-free scaffolds and scaffold-free approaches offer greater hope for the treatment of various tissue (e.g., cartilage) repair.[37] These techniques aim to produce biomimetic tissues that recapitulate the functional, structural, and biological characteristics of native articular cartilage.
GLIMPSE INTO THE FUTURE
Various experts all around the world are working relentlessly to be able to regenerate and multiple cartilage cells with the help of 3D printing. The next new innovation which would immensely help the world is “bio3Dprinting”.[38]
CONCLUSION
The biological solutions provided by the regenerative medical treatment are becoming popular and are finding favor of the patients and clinicians, over the reconstructive or replacement therapies. Regenerative medicine-based interventions are expected to become an important focus in many medical disciplines; orthopedic is one of the leading fields. Regenerative treatments aim to treat the underlying cause of the symptoms. Regenerative medical technologies, such as cell therapy, gene transfer, and tissue engineering, are expected to take orthopedics into a new era. Cell therapies have been used as a treatment for a variety of musculoskeletal pathologies including OA, cartilage defects, tendinopathies, delayed union, non-union of fractures, and avascular necrosis of femoral head and bones.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. William Murrell and Dr. Raju Vaishya are on the Editorial Board of the Journal.
References
- The journey of articular cartilage repair. J Clin Orthop Trauma. 2016;7:135-6.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr Stem Cell Rep. 2016;2:33-42.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell-and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 2009;20:646-55.
- [CrossRef] [PubMed] [Google Scholar]
- New biotechnologies for musculoskeletal injuries. Surgeon. 2019;17:244-55.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018;2018:2495848.
- [CrossRef] [PubMed] [Google Scholar]
- Prolotherapy: A new hope for temporomandibular joint pain. Indian J Pain. 2013;27:49-52.
- [CrossRef] [PubMed] [Google Scholar]
- Heterodox procedures. In: Management of Rheumatic Disorders. Boston, MA: Springer; 1983.
- [CrossRef] [Google Scholar]
- Concepts in regenerative medicine: Past, present, and future in articular cartilage treatment. J Clin Orthop Trauma. 2016;7:137-44.
- [CrossRef] [PubMed] [Google Scholar]
- Joint debridement: Surgical treatment of degenerative arthritis. Surg Gynaecol Obstet. 1941;73:1-9.
- [Google Scholar]
- Ligament and Tendon Relaxation: Treated by Prolotherapy. Vol 13. (5th ed). Oak Park, IL: Beulah Land Press; 2002. p. :6-36.
- [Google Scholar]
- The effectiveness of prolotherapy in treating knee osteoarthritis in adults: A systematic review. Br Med Bull. 2017;122:91-108.
- [CrossRef] [PubMed] [Google Scholar]
- A method of resurfacing osteoarthritis knee joints. J Bone Joint Surg Br. 1959;41:618-9.
- [Google Scholar]
- The Pridie debridement operation for osteoarthritis of the knee. Clin Orthop Relat Res. 1974;101:61-7.
- [Google Scholar]
- Microfracture for the treatment of cartilage defects in the knee joint-a golden standard? J Clin Orthop Trauma. 2016;7:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy. 2003;19:477-84.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl 2):25-32.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95.
- [CrossRef] [PubMed] [Google Scholar]
- Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tiss Eng. 1998;4:429-44.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pain management with regenerative injection therapy (RIT) In: Pain Management: A Practical Guide for Clinicians. Washington, DC: CRC Press; 2001. p. :381-402.
- [Google Scholar]
- Hope, hype, hurdles & future perspective for PRP, PRP versus hyaluronic acid injection in osteoarthritis of knee: A review article. Bio Orthop J. 2020;2:e1-12. Available from: https://www.biologicortho.com/index.php/BiologicOrtho/article [Last assessed 2020 May 20]
- [Google Scholar]
- Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: A systematic review of clinical evidence. Stem Cells Int. 2019;2019:1735242.
- [CrossRef] [PubMed] [Google Scholar]
- Leukocyte-poor platelet-rich plasma to treat degenerative meniscal tear: A case report. J Clin Orthop Trauma. 2016;7:106-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of culture-expanded, mesenchymal stem/stromal cells for the treatment of knee osteoarthritis: A systematic review protocol. J Orthop Surg Res. 2019;14:34.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: A 1-year pilot double-blinded randomized controlled trial. Am J Sports Med. 2018;46:171-80.
- [CrossRef] [PubMed] [Google Scholar]
- Demineralized bone matrix in bone repair: History and use. Adv Drug Deliv Rev. 2012;64:1063-77.
- [CrossRef] [PubMed] [Google Scholar]
- Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;22:367-74.
- [Google Scholar]
- Platelet-rich plasma for sports-related muscle, tendon and ligament injuries: An umbrella review. Blood Transfus. 2019;17:465-78.
- [Google Scholar]
- Introduction to stem cell therapy. J Cardiovasc Nurs. 2009;24:98-105.
- [CrossRef] [PubMed] [Google Scholar]
- The E. Donnall Thomas lecture: Normal and neoplastic stem cells. Biol Blood Marrow Transplant. 2008;14:849-58.
- [CrossRef] [PubMed] [Google Scholar]
- Forster ES, ed. Parts of Animals. Vol 12. Cambridge, MA: Harvard University Press; 1918. p. :167-9.
- MicroRNA in osteoarthritis: Physiopathology, diagnosis and therapeutic challenge. Br Med Bull. 2019;130:137-47.
- [CrossRef] [PubMed] [Google Scholar]
- Repair of damaged articular cartilage: Current approaches and future directions. Int J Mol Sci. 2018;19:E2366.
- [CrossRef] [PubMed] [Google Scholar]
- The upsurge in research and publication on articular cartilage repair in the last 10 years. Ind J Orthop. 2019;53:586-94.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound imaging and guidance in the management of knee osteoarthritis in regenerative medicine field. J Clin Orthop Trauma. 2019;10:24-31.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue engineering: Current strategies and future directions. Chonnam Med J. 2011;47:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Alternatives to biologics in management of knee osteoarthritis: A systematic review. Sports Med Arthrosc Rev. 2018;26:79-85.
- [CrossRef] [PubMed] [Google Scholar]
- Medical Applications for 3D Printing: Current and Projected Uses. P T. 2014;39:704-11.
- [Google Scholar]






